Research article - Volume 3 - Issue 4
Her2 Gene Amplification by Fish Technique in Ovarian Cancer Patients
Mahnaz M Kazi; Pina J Trivedi*; Dharmesh M Patel; Priya K Varma; Archana Patel
Cytogenetic Laboratory, Cancer Biology Department, the Gujarat Cancer & Research Institute, Asarwa, Ahmedabad, Gujarat, India.
Received Date : June 30, 2023
Accepted Date : July 27, 2023
Published Date: Aug 03, 2023
Copyright: © Pina J Trivedi 2023
*Corresponding Author : Pina J Trivedi, Cytogenetic lab, Cancer Biology Department, The Gujarat Cancer & Research Institute, Asarwa, Ahmedabad, Gujarat, India -380016.
Email: pina.trivedi@gcriindia.org
DOI: Doi.org/10.55920/2771-019X/1520
Abstract
Background: Human Epidermal Growth Factor Receptor-2 (HER2) gene triggers a cascade of cellular responses leading to cell proliferation, survival, and metastasis in malignant cells. Therapies targeting HER2 gene are emerging as a promising treatment option for patients with HER2 gene amplification. Hence, the present study aims to investigate the HER2 gene amplification and relationship with clinicopathological features in ovarian cancer patients.
Method: Histopathologically confirmed 37 ovarian cancer patients were included and analysed for HER2 gene amplification by Fluorescence In Situ Hybridization (FISH) using HER2/CEN 17 dual color probe kit. ASCO/ CAP guidelines were used for analysis and interpretation.
Results: HER2 gene amplification was observed in 6 patients and 3 patients has monosomy in chromosome 17 and rest 28 patients had non-amplified HER2 gene. The incidence of HER2 gene amplification was found to be 16% (6/37) in ovarian cancer patients. The amplified gene was significantly associated with older age group patients as compared to younger age group. Also, amplified HER2 gene showed a trend towards significant association in patients with high HE4. However, there was no significant correlation with rest of the clinicopathological parameters of ovarian cancer patients.
Conclusion: The incidence of HER2 amplification is found to be 16% while 8% patients had monosomy chromosome 17, which is a rare event. HER2 amplification is significantly associated with older age group and hence could be useful to treat this subset of people which otherwise would have poor response and outcome. Detection of HER2 amplification could help to identify a specific subset of the patients.
Introduction
The Human Epidermal Growth Factor Receptor (HER) family member - HER2 is a transmembrane tyrosine kinase receptor that triggers a cascade of cellular responses leading to cell proliferation, survival, and metastasis [1,2]. HER2 expression is often observed to be dysregulated in various malignancies such as those of breast, ovarian, lung, prostate, colorectal and gastric cancer [3-8]. Overexpression of HER2, commonly by gene amplification is observed in 15-30% of breast cancers, wherein an anti-Her2/neu-therapy administering Trastuzumab is directed against the extracellular domain of the Her2/neu protein and reveals a significant anti-tumour activity [9,10]. It is an established therapeutic target in breast and gastric carcinomas [11,12].
HER2 amplification is also observed in ovarian cancer with an incidence of 20-30%. Moreover, ovarian cancer patients with HER2 overexpression were found to be associated with poor survival. In addition, concern with Epithelial Ovarian Cancer (EOC) which is responsible for more mortality than any other gynecologic malignancy, is lack of a screening test, absence of specific symptoms early in the disease course as well as the development of acquired drug resistance in patients. Thus, application of trastuzumab, can result in increased overall survival of such patients with amplified HER2 gene. As administration of therapeutic agent depends on the detection of HER2 gene amplification, precise estimation of HER2 levels in EOC is required. Based on this, the present study aimed to evaluate the ovarian cancer patients for the amplification of HER2 gene by Fluorescence In-Situ Hybridization (FISH) so that the patients could be treated accordingly.
Material and Methods
Patients
In the present retrospective study, a total of 37 untreated histologically confirmed ovarian cancer patients registered at The Gujarat Cancer & Research Institute were enrolled. The study was approved by the Institute’s Ethics Committee Board and general consent forms were obtained from all the patients. Detailed clinical and histopathological record of the patients such as age at diagnosis, gender, tumor differentiation, lymph node involvement etc. was obtained from the institute’s medical records. The clinicopathological characteristics of the enrolled patients are enlisted in Table 1. All ovarian cancer patients in the present study were of epithelial type.
Fluorescence In-Situ Hybridization (FISH)
FISH analysis was carried out on formalin fixed paraffin-embedded tumor tissue blocks of the enrolled ovarian cancer patients using commercially available ZytoLight SPEC ERBB2 /CEN 17 Dual Color Probe Kit (ZytoVision GmbH, Germany). The probe consists of two fluorochrome-labelled DNA probes - ERBB2 (HER2) labelled with Spectrum Green and CEN17 probe labelled with Spectrum Orange. FISH assay was performed as per the manufacturer’s instructions. Pre-treatment included deparaffinization of tumor tissue sections followed by dehydration with alcohol series (100%, 90%, 70%) and incubation in Pre-Treatment Solution (PT1) at 900 C. Protease action was done by applying Pepsin Solution (ES1) to the tissue section. Subsequently, probe was applied prior to denaturation and hybridization process which was carried out overnight at 37°C in a humidifying chamber. Next day, post-hybridization washes were given, and slides were counterstained with DAPI. The slides scanning and capturing were done using OLYMPUS BX61 fluorescent microscope (OLYMPUS BX61, Japan). Images were captured at a magnification of 100X, Total 20 randomly selected invasive tumor cells were evaluated for interpretation. The ratio of HER2 signals to centromere CEN17 signals were calculated and ASCO/CAP guidelines were used for analysis and interpretation of results.
Statistical analysis
The statistical evaluation of the data was carried out using SPSS Inc. version 20 software. Two-tailed chi square test and spearman’s correlation method was used to correlate the HER2 gene expression with various clinicopathological characteristics of ovarian cancer patients. P≤0.05 was considered to be statistically significant.
Results
Clinicopathological parameters of Ovarian Cancer patients
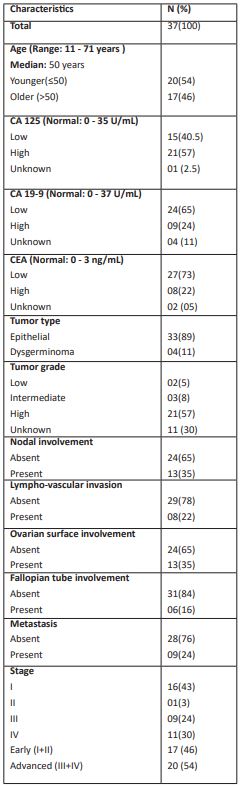
HER2 gene expression was analyzed in 37 pre-treated ovarian cancer patients using formalin fixed paraffin embedded tissue samples. As shown in Table 1, ovarian cancer patients enrolled in the study were in the age group of 11 to 71 years and the median age of patients was 50 years. Median age was used as a cut off to categorize the patients into younger (≤50) and older age group (>50). Accordingly, 20 (54%) patients were in younger age group and 17 (46%) patients were in older age group. Cancer Antigen (CA)-125 level normal range is 0 - 35 U/mL and accordingly 15 (40.5%) patients had normal values of CA-125 and 21 (57%) patients had high level of CA-125. Normal range of Carbohydrate Antigen (CA) 19-9 level is 0 - 37 U/mL and accordingly 24 (65%) patients exhibited normal values of CA19-9 and 9 (24%) patients had high level of CA 19-9. With respect to Carcinoembryonic Antigen (CEA) (normal range: 0 - 3 ng/mL), 27 (73%) patients had normal values and 8 (22%) patients had high values of CEA.
Out of 37 patients, the type of ovarian cancer in 33 (89%) patients was epithelial type and 4 (11%) patients were of dysgerminoma tumor type. Of total patients, 2 (5%) patients belonged to low grade tumor, 3 (8%) were of intermediate grade and 21 (57%) were of high-grade tumor. The tumor grade of the remaining patients was not known. In this study, nodal involvement by tumor was found in 13 (35%) patients while 24 (65%) patients did not show nodal involvement. Lympho-vascular invasion was present in 8 (22%) patients and absent in 29 (78%) patients. Ovarian surface involvement by tumor was present in 13 (35%) patients and absent in 24 (65%) patients. Fallopian tube involvement of tumor was observed in 6 (16%) patients and absent in 31 (84%) patients. There were 16 (43%) patients belonging to stage I, 1 (3%) patient was in stage II, 9 (24%) patients in stage III and 11 (30%) patients were in stage IV.
Table 1: Clinico-pathological characteristics of Ovarian Cancer patients.
HER2 gene status
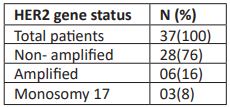
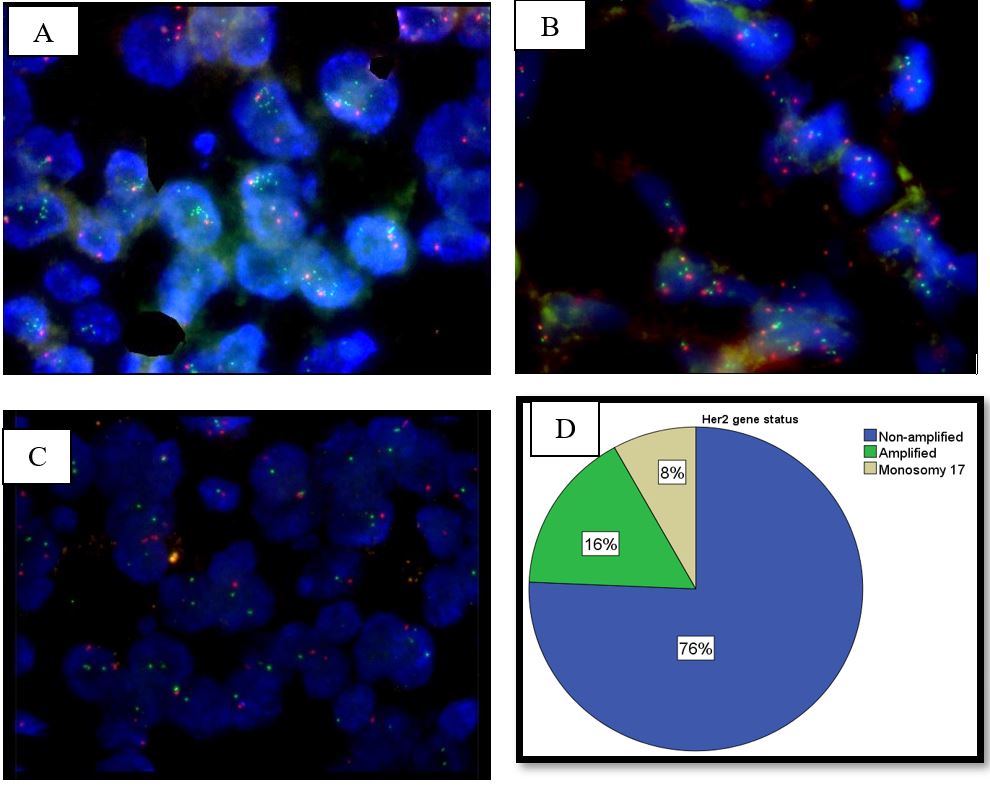
The observed signaling pattern for ERBB2/CEN 17 dual color probe in the present study leads to following interpretations: out of 37 (100%) patients, 28 (76%) patients showed OOGG signal pattern in a cell and had ratio of HER2 gene/CEN 17 signals < 2 as well as HER2 copy signals < 4 / cell. Hence, these patients were considered non-amplified for HER2 gene. There were 6 (16%) patients who showed multiple green signals in a cell and had ratio of HER2 gene/CEN 17 signals ≥ 2 as well as HER2 copy signals ≥ 4/cell. Hence, these patients were considered amplified for HER2 gene. In addition, 3 (8%) patients showed OG signal pattern indicating monosomy of chromosome 17 (Table 2, Figure 1).
Table 2: HER2 gene status
Figure 1: (A) FISH results showing amplified HER2 gene.
(B) FISH results showing non amplified HER2 gene.
(C) FISH results showing monosomy of chromosome 17.
(D) Graphical representation of HER2 gene amplification of ovarian cancer patients.
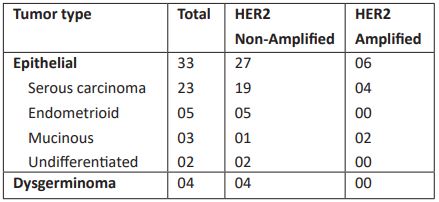
Out of total ovarian cancer patients, 33 patients belong to epithelial type, in which 27 patients had non-amplified HER2 gene and 6 patients had HER2 gene amplification. Of 33 epithelial type ovarian cancer, 23 were of serous carcinoma, 5 belonged to endometrioid type, 3 were of mucinous type and 2 patients had undifferentiated tumor type. Four patients of serous carcinoma were HER2 amplified and 2 of mucinous carcinoma were HER2 amplified. There were 4 patients with dysgerminoma, and all were HER2 non-amplified (Table 3).
Table 3: Ovarian Cancer types and HER2 gene status.
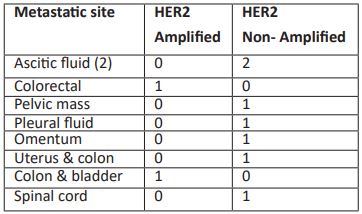
Out of 37 patients, 28 (76%) patients did not show metastasis while 9 (24%) patients were metastasized in which HER2 amplification was noted in 2 patients. No correlation was observed between HER2 status and metastasis. Among 9 metastasized patients 2 patients had metastasis in ascitic fluid both were negative for HER2 status, and one patient had metastasis in colorectal which was HER2 negative. 5 patients of Pelvic mass, pleural fluid, omentum, uterus & colon, and spinal cord all are negative for HER2 status. Colon & bladder metastasis observed in 1 patient which was negative for HER2 status.
Table 4: HER2 gene status in different metastatic sites.
Correlation of clinico-pathological characteristics of ovarian cancer patients with HER2 gene status
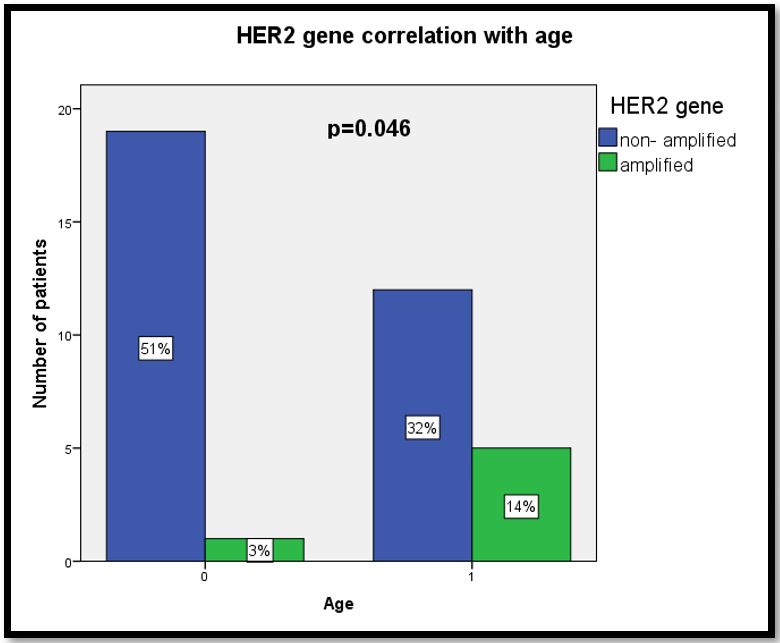
As shown in Table 5, clinico-pathological characteristics were correlated with HER2 gene status to analyse possible association of these characteristics in ovarian cancer. Amplification of HER2 gene was significantly associated with patients in older age group (29%) as compared to those in younger age group (71%; χ2= 4.031,r= 0.330, p=0.046) (Figure 2).
Preponderance of amplified HER2 gene was observed in patients with high grade ovarian tumor (19%) as compared to those with low grade (0%) and intermediate grade ovarian tumors (0%), but without statistically significant correlation. Similarly, amplified HER2 gene was observed in patients with nodal involvement (23%) as compared to absence in nodal involvement patients (12.5%), but without statistically significant correlation. Further, higher incidence of amplified HER2 gene was observed in patients with lympho- vascular invasion (25%), advanced stage patients (20%) and patients with metastasis (22%) as compared to those without lympho-vascular involvement patients (14%), early-stage patients (12%) and patients without metastasis (14%), respectively, however the correlation was not statistically significant.
Table 5: Correlation of clinico-pathological characteristics of ovarian cancer patients with HER2 gene status.
Figure 2: Graph for HER2 correlation with age.
Significant association of amplified HER2 gene in patients with older age as compared to patients with younger age (p=0.046).
Discussion
Activated HER2 pathway initiates intracellular signaling pathways, including the MAPK and PI3K-Akt pathways [13]. Dysregulation of HER2 signaling in the ovary occurs, frequently due to overexpression or mutations in HER family members and is often linked to the growth and proliferation of ovarian tumors. Overexpression of HER2 is one of the most common and frequent mechanisms, by which HER2 signaling is activated in oncogenesis. Different studies have reported varied incidence rates of HER2 amplification in ovarian cancer, ranging from approximately 5% to 30%, determined by several methods [14]. In the present study, a total of 37 pre-treated ovarian cancer patients were studied for HER2 gene amplification by FISH method. The study was conducted to identify incidence of HER2 amplification in ovarian cancer patients and its correlation with clinico-pathological parameters. The present study observed HER2 gene amplification rate to be 16% (06/37) in ovarian cancer patients. This is in accordance with the study by McAlpine et al. wherein HER2 gene amplification was observed in 18.2% (6/33) of ovarian mucinous carcinomas [15]. Moreover, various studies have reported HER2 gene amplification in 27.3%, 10.41% and 66% of ovarian cancer patients [16,14,17]. Such difference in incidence rates of HER2 amplification could be due to use of different methods of detection. Further, a rare event of monosomy chromosome 17 was observed in 8% (3/37) of ovarian cancer patients in the present study. Similarly, Brunelli et al has also observed monosomy of chromosome 17 in 2.5% of breast cancer patients [18]. Moreover, Nakopoulou et al reported that monosomy 17 also had been associated with nodal metastasis [19], while no such correlation was observed in the present study.
Further, in the present study, age of the enrolled ovarian cancer patients ranged from 11 to 71 years with median age of 50 (p=0.046). Similar median age of 50 years was reported by Cai et al in their study [20]. Also, we observed a significant association of amplified HER2 gene with older age group patients (29%) as compared to younger age group (71%), implying that HER2 amplification might be related to aggressiveness of the disease. However, several other studies did not find significant association between HER2 amplification and age of the patient [20-22].
The present study did not find significant difference between HER2 status in ovarian cancer patients and tumor stage, tumor grade and type. Similarly, various other studies too did not find any significant association of HER2 gene with tumor stage, histological type, and histological grade of ovarian cancer patients [21,23] and breast cancer patients [24,25]. In addition, the present study observed that HE4 marker showed a trend towards significance with amplified HER2 gene. Similarly, [26] observed strong association between HER2 status and HE4 in breast cancer patients. These may be commented as HE4 expression rises in patients with HER2 amplification [26]. Concluding, although not significantly associated with any other parameters, the present study observed a significant higher incidence of HER2 amplification in older age group. Hence, this subset of patients could be stratified and treated with anti-HER2 therapies to get a better response and outcome.
References
- Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995; 19: 183-232.
- Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995; 10: 1813-21.
- Murthy SS, Sandhya DG, Ahmed F, et al. Assessment of HER2/Neu status by fluorescence in situ hybridization in immunohistochemistry-equivocal cases of invasive ductal carcinoma and aberrant signal patterns: A study at a tertiary cancer center. Indian Journal of Pathology and Microbiology. 2011; 54: 532.
- Shang AQ, Wu J, Bi F, et al. Relationship between HER2 and JAK/STAT-SOCS3 signaling pathway and clinicopathological features and prognosis of ovarian cancer. Cancer biology & therapy. 2017; 18: 314-322.
- Pillai RN, Behera M, Berry LD, et al. HER2 mutations in lung adenocarcinomas: A report from the Lung Cancer Mutation Consortium. Cancer. 2017; 123: 4099-4105.
- Sharifi N, Salmaninejad A, Ferdosi S, et al. HER2 gene amplification in patients with prostate cancer: Evaluating a CISH-based method. Oncology letters. 2016; 12: 4651-4658.
- Li Q, Wang D, Li J, Chen P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC cancer. 2011; 1: 1-6.
- Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: An update. World Journal of gastroenterology. 2016; 22: 4619.
- Slamon DJ , Leyland-Jones B, Shak S, Fuchs H, Paton V, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001; 344: 783-792.
- Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. Journal of clinical oncology. 2002; 20: 719-726.
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010; 376: 687-697.
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011; 365: 1273-1283.
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: Receptor heterodimerization in development and cancer. The EMBO journal. 2000; 19: 3159-3167.
- Alzeyadi M, Imarah AA, Khayoon SQ, Alhamadani IM. Cytogenetic Analysis of HER2 in Ovarian Cancer Patients by Fluorescence in Situ Hybridization. European Journal of Engineering Science and Technology. 2020; 3: 1-7.
- McAlpine J, Wiegand K, Miller M, Adamiak A, Koebel M, et al. HER2 Overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. Gynecologic Oncology. 2010; 116: 593-594.
- Verri E, Guglielmini P, Puntoni M, Perdelli L, Papadia A, et al. HER2/neu oncoprotein overexpression in epithelial ovarian cancer: Evaluation of its prevalence and prognostic significance. Oncology. 2005; 68: 154-161.
- Ross JS, Yang F, Kallakury BV, Sheehan CE, Ambros RA, et al. HER-2/neu oncogene amplification by fluorescence in situ hybridization in epithelial tumors of the ovary. American journal of clinical pathology. 1999; 111: 311-316.
- Brunelli M, Nottegar A, Bogina G, Caliò A, Cima L, et al. Monosomy of chromosome 17 in breast cancer during interpretation of HER2 gene amplification. American journal of cancer research. 2015; 5: 2212.
- Nakopoulou L, Giannopoulou I, Trafalis D, Gakiopoulou H, Keramopoulos A, et al. Evaluation of numeric alterations of chromosomes 1 and 17 by in situ hybridization in invasive breast carcinoma with clinicopathologic parameters. Applied Immunohistochemistry & Molecular Morphology. 2002; 10: 20-8.
- Cai Y, Wang J, Zhang L, Wu D, Yu D, et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Medical oncology. 2015; 32: 1-6.
- Tuefferd M, Couturier J, Penault-Llorca F, Vincent-Salomon A, Broet P, et al. HER2 status in ovarian carcinomas: A multicenter GINECO study of 320 patients. PloS one. 2007; 2: e1138.
- Gupta S, Mehra M, Khattri J, Madhvi M. A study of her-2/neu oncogene expression in benign and malignant ovarian tumors. 2020.
- Grover A, Mohanty M, Dash K. HER-2 neu expression in surface epithelial ovarian tumors and its relationship with clinic-pathological parameters: A pilot study. International Journal of Reproduction, Contraception, Obstetrics and Gynecology. 2021; 10: 1363-1368.
- Cong TD, Thanh TN, Phan QA, Thi AP, Tran BS, et al. Correlation between HER2 expression and clinicopathological features of breast cancer: A cross-sectional study in Vietnam. Asian Pacific Journal of Cancer Prevention: APJCP. 2020; 21: 1135.
- Yadav R, Sen R, Chauhan PR. ER, PR, HER2/NEU status and relation to clinicopathological factors in breast carcinoma. Int J Pharm Pharm Sci. 2016; 8: 287-290.
- Akoz G, Diniz G, Ekmekci S, Ekin ZY, Uncel M. Evaluation of human epididymal secretory protein 4 expression according to the molecular subtypes (luminal A, luminal B, human epidermal growth factor receptor 2-positive, triple-negative) of breast cancer. Indian Journal of Pathology and Microbiology. 2018; 61: 323.