Research Article - Volume 3 - Issue 4
Occult HBV infection among chronic hemodialysis patients and its role in viral transmission
Qusay Abdoh1,2,4,,*, Mohammad Alnees 1,2,3,*, Abdalaziz Darwish1, Duha Najajra1, Mahdi Awwad1,Laith Abu Snouber5,Layan Melhem1,Maamooun Rapya1, Adham Abu Taha1, Zakaria Hamdan1,Nizar Abu Hamdeh1, Rifat Safadi1
1Department of Medicine, Faculty of Medicine & Health Sciences, An-Najah National University, Nablus, Palestine.
2equally distributed
3 Harvard Medical School Postgraduate Medical Education,Global Clinical Scholars Research Training program, Boston, US.
4Department of Internal Medicine,GI and Endoscopy unit , An-Najah National University Hospital, Nablus, Palestine.
5Department of pediatrics, Makassed Islamic Charitable Hospital, Mount of Olives, Jerusalem, Palestine.
Received Date : July 07, 2023
Accepted Date : August 10, 2023
Published Date: August 22, 2023
Copyright:© Qusay Abdoh 2023
*Corresponding Author : Qusay Abdoh, An-Najah Hospital, Nablus,West Bank, occupied Palestinian territory.
Email: Qusaygi@yahoo.com
Mohammad Alnees, Department of Medicine, Faculty of Medicine & Health Sciences, An-Najah National University, Nablus, Palestine. Harvard Medical School Postgraduate Medical education,Global Clinical Scholars Research Training program, Boston,US.
Email: a2011z2012z2013@gmail.com
DOI: Doi.org/10.55920/2771-019X/1528
Abstract
Introduction: Hepatitis screening pose a vital role among hemodialysis patients, starting from HBsAg to advanced screening using advance technique such as PCR. Occult Hepatitis B infection can be defined as those who are HBsAg negative, HBcAbs positive, and HBV DNA PCR positive. There is a gap of knowledge regarding Occult hepatitis B infection among hemodialysis patients world wild. In Palestine, there is a need to study the Hepatitis screening practices in hemodialysis unites.
Methods: this study was conducted among 266 hemodialysis patients in Al Najah National University hospital. The inclusion criteria were hemodialysis patients who were HBsAg negative at baseline screening. The HBcAb test was done for all the recruited patients, while the HBV DNA PCR was done only for patients who were HBcAb positive.
Results: After six months, all patients who were found HBcAb negatives at basleine, were tested again for HBcAb, among those who were HBcAB positive at six month the HBV DNA PCR was done. The results: A total of 249 patients were included in the study, the mean age was (56.8±14.5) years. The overall prevalence of occult hepatitis B infection among the patients at the end of the study was 4.7% (4/85) but only 4.5% (12/266) of the hemodialysis patients were positive for HBsAg. About 34.1% (85/249) of the hemodialysis patients were anti-HBc positive. Further analysis was done employing Chi Square test. Although the association between the hemodialysis sessions with HBcAb test was not significant at baseline; the analysis revealed significant association between being HBcAb positive and hemodialysis session (p<0.05) after six month. Similar findings were reported by dividing the hemodialysis sessions into two groups according to session days at p< 0.05 using the same statistical test.
Conclusion: Considerable incidence of Occult Hepatitis B infection among hemodialysis patients was reported, which necessitate more screening tests and control policies. Which may indicate that HBsAg assay is not sufficient for accurate screening of HBV infection among hemodialysis patients.
Keywords: Occult hepatitis B virus; HBV DNA; infection; viral transmission; Hemodialysis.
Abbreviations
Anti-HBe: Antibodies against Hepatitis B envelope antigen
Anti-HBs: Antibodies against Hepatitis B surface antigen
CDC: Centers for Disease Control and Prevention
CHB: Chronic Hepatitis B
DNA: Deoxyribonucleic acid
EPI: Expanded programme on immunization
ESRD: End stage renal disease
HBcAbs/Anti-Hbc: Total Antibodies against hepatitis B core antigen
HBeAg: Hepatitis B envelope antigen
HBsAg: Hepatitis B surface antigen
HBV: Hepatitis B virus
HCV: Hepatitis C virus
Introduction
Hepatitis is an infection-related liver inflammation that has a high rate of morbidity and mortality. High levels of infection can spread from through blood transfusions, sexual activity, and vertical routes. patients with end-stage renal disease are more likely to have HBV and HCV infections. These blood-borne which are a source of higher morbidity and mortality may be more infectious in HD patients than in the general population [1]. The European Association for the Study of the Liver (EASL) reports that roughly 33% of the global population shows serological evidence indicating that they have had either current or previous hepatitis B virus (HBV) infections. Furthermore, more than 350 million individuals may be suffering from persistent HBV infections [2].
Hepatitis B virus (HBV) genotypes and subgenotypes have distinct geographical distributions and influence a number of clinical disease features and responses to treatment. These genotypes and subtypes have distinct geographic patterns, transmission pathways,and advancement [3]. Therefore, the sensitivity of the tests used to identify HBsAg and HBV DNA forms the basis for a reliable diagnosis of OBI. Many commercially available HBsAg assays have a 0.05 IU/mL lower limit of detection (LLOD), and it has been demonstrated that up to 48% of samples that test negative using these assays turn positive when tested using more sensitive HBsAg tests (LLOD of 0.005 IU/mL) [4].
Also, Most patients with undetectable HBsAg also have undetectable HBV DNA. Despite the absence of HBsAg, some patients continue to replicate HBV DNA at a low level. When viral protein expression is absent yet persistently low levels of HBV DNA are present, this pattern is known as occult hepatitis B infection (OBI).The majority of people with OBI have anti-HBc and, less frequently, anti-HBs positivity [5].
In 2016, the prevalence of chronic hepatitis B virus (HBV) infection was estimated at 3.9% (3.4%-4.6%) among 292 million people . There are several indicators of past and present HBV infection and vaccination. Hepatitis B surface antigen positive information (HBsAg) Estimates are discussed here as a useful sign of an active infection. Only 3.5% of HBV infections occur in high-income populations. the remaining nations are in low- and middle-income nations [6]. And In Palestine, over 1350 patients receive dialysis in 12 different health centers distributed other the West-bank alone. Moreover, Palestine is considered an endemic area for Hepatitis B infection with infection rate 0.51/100.000 and Hepatitis B carrier state of 19.9/100.000. Hence it’s important to look into both hemodialysis and Hepatitis B infections in Palestine with interest [7].
Due to their exposure to blood products and weakened immune systems, patients with end-stage renal illness are susceptible to catching the hepatitis B virus (HBV). ongoing surveillance is essential to stopping the spread of this powerful DNA virus. Maintaining a safe environment for dialysis to take place requires regular assessment of serologic indicators together with adequate isolation and decontamination techniques. In the hemodialysis population, vaccination response rates are known to be less than ideal [8]. A multifaceted strategy is necessary to prevent HBV transmission in the HD environment. The significance of effective infection control procedures for the prevention of HBV infections cannot be emphasised enough. Correct isolation methods implementation and universal vaccination of vulnerable patients both contribute to the prevention of HBV transmission [9]. Considering the prevalent nature of hepatitis B viral infections - specifically within the population of individuals enduring end-stage renal disease - concomitant with the infection's endemic qualities encompassing throughout Palestine, our persuasive discoveries may potentially contribute significantly towards augmenting the sophistication of infection containment and prevention strategies, thereby diminishing the corresponding morbidity and mortality rates essentially linked with the above-mentioned viral affliction.
Subjects and methods
Study Design
The current design is prospective cohort study with an objective to determine the incidence of occult hepatitis B and to determine the viral transmission among hemodialysis patients at Al Najah National University Hospital. The study was conducted in two phases, phase one (September 2017) started with subjects screening for HBsAg (Hepatitis B surface antigen), HBsAb (Hepatitis B surface antibody) and HBcAb (Hepatitis B core antibody) for all subjects. Subjects’ samples who were HBsAg, HBsAb negative and HBcAb positive were stored for further analysis for HBV DNA. Phase two was conducted after six month (March 2018) of the subjects’ recruitment. In this phase subjects who were found HBcAb negative at baseline were examined again for HBcAb. Among those who were found HBcAb positive at six month in addition to the stored samples, further assessment for HBV DNA was done (Figure 1).
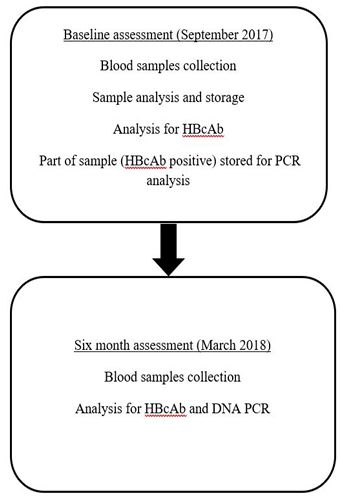
Figure 1: Study design flow chart.
Subjects selection
a.Sampling method
The subjects were selected by total sampling, in which all of the patients who met the inclusion criteria were invited to join the study and sign the consent form.
b.Inclusion criteria
All hemodialysis patients who receive the treatment and follow up at the hemodialysis unit at Al Najah National University Hospital.
c.Exclusion criteria
Patients who were HBsAg positive and patients who have hepatitis C infection in addition to patients who refused to join the study.
Blood sample collection and analysis
a.blood sample collection and storing
The baseline assessment was started by collecting 20 ml of venous blood from each participant by the dialysis unit staff. The blood samples were sent to Al Najah National University Medical laboratory. The blood samples were aliquoted into different Eppendorf tubes. Part of the blood samples were directly used in quick analysis for HBcAb, and the rest of blood was stored at -80 C and transferred to the Arab American University Medical Laboratory for PCR analysis
b.Analysis for HBcAb
Anti-HBc antibody test is a competitive enzyme immunoassay in which anti-HBc antibodies from specimens compete with a constant amount of Horseradish Peroxidase (HRP) conjugated anti-HBc antibody for a limited number of HBcAg coated on the microwells together with polyclonal antibodies against HBcAg as catcher for HBcAg.
After preparing the equipments and materials needed (Microwell holder, humidified box and 37oC incubator, microtiter plate washer and the EIA reader), all reagents were allowed to reach room temperature, then 10 ul of plasma were diluted with 500 ul of diluted washing solution and 50 ul of diluted specimen were dispensed into each well.
One drop (50 ul) of Positive Control as well as Negative Control was dispensed into respective wells, then one drop (50 ul) of Enzyme Conjugate was added to each well and mixed gently by swirling the microtiter plate on flat bench for 2 min. The microtiter plate was placed into a humidified box and incubated at 37o C for 60 min.
Each well was washed 6 times by filling each well with diluted wash buffer, then inverting the plate vigorously to get all water out and blocking the rim of wells on absorbent paper for a few seconds. One drop (50 ul) of each substrate solution was added to each well and mixed gently and incubated at room temperature for 15 min.
Visual inspection of the color reaction in each well was done or by adding 1 drop (50 ul) of stop solution to each well to stop the color reaction. We blanked EIA reader with a blank control well and then we read the optical density (OD) values of all samples at 450 nm.
The presence or absence of HBcAb is determined by comparing the absorbance value of the specimen to a cut-off value. The cut-off value is calculated from Negative and Positive Controls:

Positive Result: Specimens whose absorbance value is equal to or less than the cut-off value are to be considered reactive for HBcAb.
Negative Result: Specimens with absorbance value greater than the cut-off value are considered negative for HBcAb.
c.Analysis for PCR
Viral DNA was extracted from 200 µl of the 85 serum samples, using the QIAamp viral DNA extraction kit according to the manufacturer instructions (Qiagen, Hilden, Germany). The final volume of the DNA elute was 35μl.
The HBV-DNA was amplified using nested PCR with two primer sets targeting the viral polymerase gene of the HBV genome described previously by (Selabe et al. 2007). In brief, the nested PCR was carried out as follows: The 1st PCR round, was carried out in 25μl- reaction mixture containing 4 μl viral DNA extraction, 10 pmol of the outer primers (HBV-F1; 5’-GTC TGC GGC GTT TTA TCA-3’ and HBVF-R1; 5’-GGA GTT CCG CAG TAT GGA TCG G-3’) and 12.5 μl of PCR Reddy master mix (Thermo Scientific). In the second PCR round, 2 µl from the first round was further amplified in 25 μl reaction mixture containing 10 pmol of the inner primers (HBV-F2; 5’-GGTATG TTG CCC GTT TGT CC-3’ and HBV-R2; 5’-GGC GAG AAA GTG AAA GCC T-3’) and 12.5 μl of the PCR master mix (Thermo Scientific). In each PCR run, negative (nuclease-free water) and positive controls were included. Five-seven microliters of PCR product were analyzed by electrophoresis on a 2% agarose gel containing ethidium bromide and were visualized using the Gel Doc System 2000 (Bio-Rad Laboratories-Segrate, Milan, Italy). A band of 647-bp indicated a positive result.
d.Occult test
Specimens declared to be reactive for HBcAb and tested positive for HBV DNA PCR are considered to be positive for Occult Hepatitis B infection.
Statistical analysis
All statistical analysis was carried out using the Statistical Package for Social Silences (SPSS) software version 22. An alpha level of (0.05) was considered for all the statistical tests used in the study. Two sided p values of (0.05) and (80%) power were considered to be statistically significant. The data were analyzed according to variable types.
Descriptive analysis for the screening test incidence was done to determine the percentage and the data were presented in number and percentage. The changes in the incidence of HBcAb was calculated by recoding the variable to (change or didn’t change). The association between the hepatitis tests (HBcAb, HBV DNA PCR) at baseline and after six months was done using Chi square tests, as the variables all are categorical. The association between the incidences of HBcAb positive occurrence with the hemodialysis sessions was analyzed using Chi square tests.
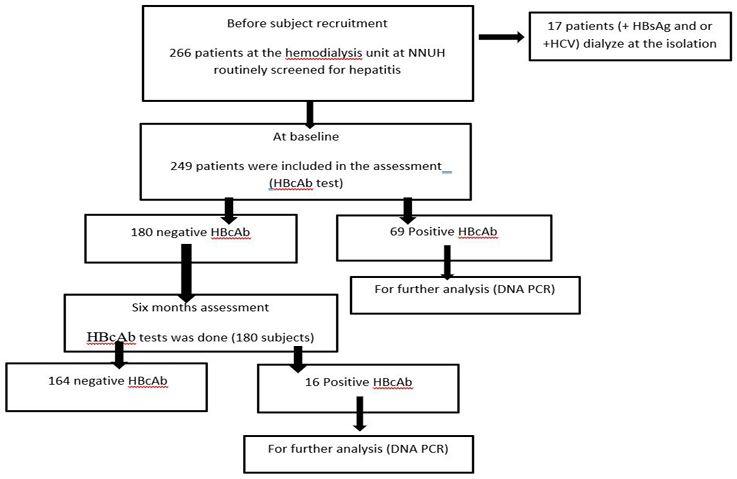
Table 1: Subjects sociodemographic characteristics of the participants presented in number and percentage.
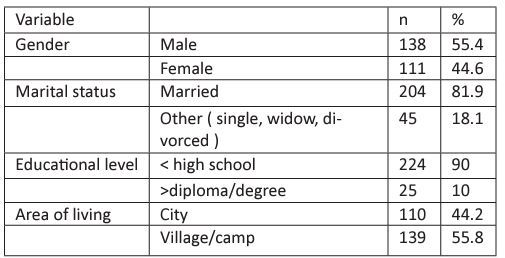
Table 2: Medical history of the participants presented in number and percentage
Table 3: Dialysis related data.
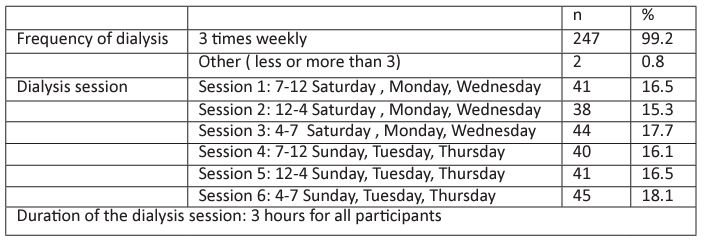
Table 4: Hepatitis screening tests.
Results
The purpose of this study was to determine the incidence of Occult Hepatitis B and to determine the viral transmission among hemodialysis patients at Al- Najah National University. This chapter presents the results of the study participants sociodemographic status, medical history, advanced hepatitis screening tests (HBcAb, DNA PCR and occult hepatitis B state). The subjects’ recruitment began in September 2017 till March 2018.
Subjects recruitments
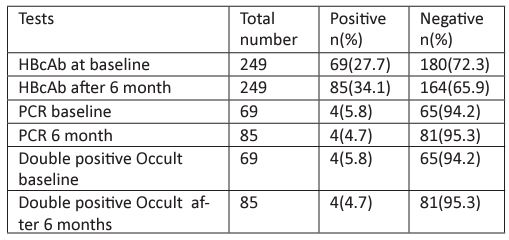
As shown in figure 2, all subjects in the hemodialysis unit were routinely screened for HBs and HCV. 249 subjects were included in the baseline assessment for HBcAb, among those who were HBcAb positive (69 patients), only 4 were positive for HBV DNA PCR. After six months, a total of 180 patients who were HBcAb negative at baseline, the test was done again, 16 of the patients showed positive HBcAb test. For the 16 patients further analysis was done to assess for HBV DNA PCR and the results were negative for all.
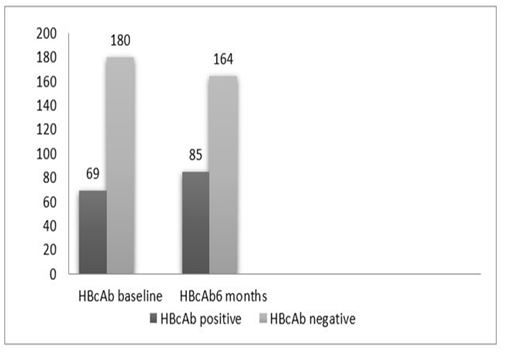
Figure 3 (a): HBcAb according to phase.
* Significant at p<0.01 using Chi Square tests
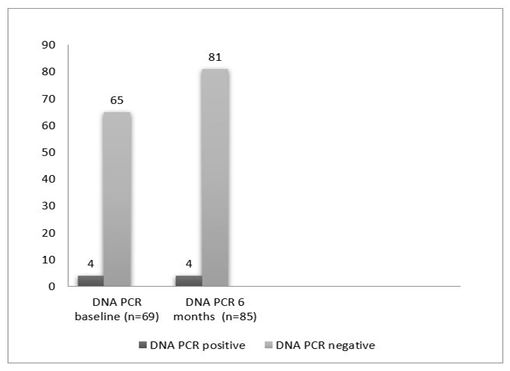
Figure 3 (b): PCR test according to phase.
Table 5: The association between hemodialysis session and HBcAb test at baseline
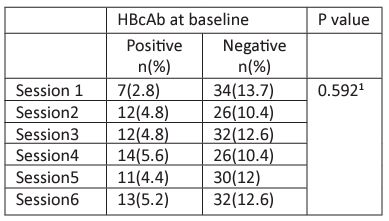
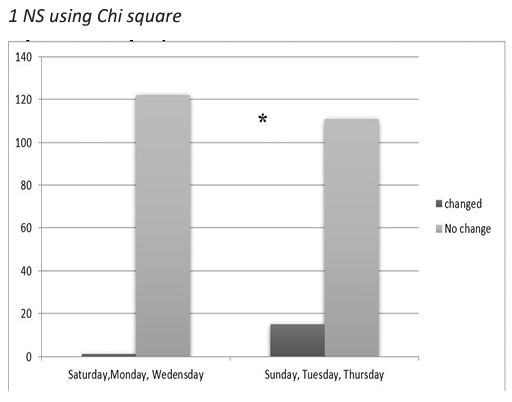
Figure 4: HBcAb changes according to session days.
* Significant at P<0.01 using Chi square tests.
Patients characteristics
a. Sociodemographic characteristics
The study finally involved 249 patients, 138(55.4%) men and 111(44.6%) women; the mean age was (56.8±14.5) years. As shown in table 1, the majority of the patients were married (81.9%), level of education was under high school level (90%). Only (44.2%) live in cities while the rest (55.8%) live either in villages or camps.
b. Medical history
As shown in table 2, around 40% of the subjects have diabetes mellitus; more than 50% of the patients have hypertension. While 32% of the patients have both Diabetes mellitus and hypertension. Among the total patients, 22(8.8%) patients have undergone kidney transplantation.
c.Dialysis related information
Majority of the patients (99.2%) go for dialysis 3 times weekly. The duration of the dialysis session usually ranges 2-3 hours. The patients were almost evenly distributed in 6 sessions; 3 sessions allocated in Saturday, Monday, Wednesday. The other 3 sessions allocated in Sunday, Tuesday and Thursday, following the hospital arrangements.
Hepatitis screening profile
Table 4 summarizes the tests that have been done for the participants, a total of 249 patients who screened for HBcAb, 69(27.7%) were HBcAb positive at baseline. Second assessment after 6 months was done for the patients who were HBcAb negative at baseline, the result showed that 16 more patients became HBcAb positive (Figure 3.a). Using Chi square tests, the increase in the number showed significant changes (P<0.05).
Table 6: The association between hemodialysis session and HBcAb test at 6 months.
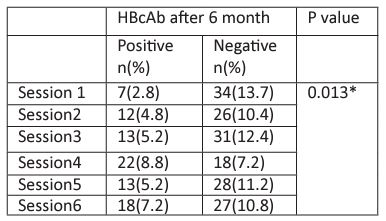
* Significant at P<0.05 using Chi square tests
The results of HBV DNA PCR were positive among 4 (5.8%) patients (patients who were HBcAb positive at baseline). The second assessment of HBV DNA PCR was done after 6 months only for the patients who became HBcAb positive (16 patients). Among those 16 patients no patients showed positive PCR (Figure 3.b).
Hepatitis B screening profile
The association between the screening tests and the dialysis session
The association between the hemodialysis sessions at baseline with HBcAb test was not significant using Chi square tests as shown in Table 5. Interestingly, the result was different after 6 months; there was significant association between being HBcAb positive and hemodialysis session. Similar findings were reported by dividing the hemodialysis sessions into two groups according to session days as shown in Figure 3.
Discussion
A-Overt Hepatitis B infection.
The hepatitis B virus (HBV) is a significant global health issue. The reported prevalence in the general population for the year 2015 was 3.5%. HD patients are at risk for HBV infection because they often receive blood transfusions, have impaired immune systems, and have invasive procedures involving blood manipulation. As a result, they ought to undergo serology testing, and all seronegative patients should receive vaccinations. Despite this, some isolated outbreaks of HBV infection continue to be reported among HD patients in developed countries [10]. In Palestine, the prevalence of HBV was 2.98% in 2020, which is higher than the prevalence in 2005 which was 1.8% according to Palestinian Health Information Centre [11].In this study, the prevalence of overt HBV (HBsAg positive) in the largest hemodialysis unit in northern Palestine – NNUH/Nablus was shown to be 4.5% which is significantly high compared with normal population. This prevalence is lower than that found in a study done among hemodialysis units in the two northern Palestinian districts, Jenin and Tulkarem (8.2%) [12].
In comparison to some Arab countries, the prevalence in this study is more than that in Morocco (1.79%) and Saudi Arabia (3.24%). In Jordan is(2.4%) and the prevalence is lower in Egypt (1.4%). Regarding other parts of the world, in France for example, the prevalence of HBV was (0.65%), which makes the country characterized by a low endemicity estimated [11,13].
B-Occult Hepatitis B in NNUH dialysis unit
Our study showed that Occult HBV infection (defined as those who are HBsAg negative, HBcAbs positive, and PCR positive [14, 15].) was common among hemodialysis patients in NNUH (4.7%), a very similar study conducted in Palestine in 2014 recruited patients from two north districts showed prevalence of 12.5% [12]. however, as there is no well accepted universal definition for Occult hepatitis B infection, this study considered another definition (defined as the presence of HBV DNA in the liver and/or serum with undetectable HBsAg with the available assays, and with or without the presence of HBV antibodies outside the acute phase window period ( which may explain the difference in the results)[16]. Another possible explanation for this variation is the difference in the degrees of implementation of universal precautions taken in each of the investigated hemodialysis units in the two studies.
The wide range of occult hepatitis B infection prevalence among hemodialysis patients worldwide in general, and the variation in prevalence between nearby countries could be attributed to several factors including the sensitivity and specificity of the PCR assay, characteristics of study population, and the difference in geographical endemicity of HBV infection.
C-Anti-HBc results significance
As for anti-HBc results, 27.7% were HBcAb positive at baseline and second assessment after 6 months for the patients who were HBcAb negative at baseline showed that 16 more patients became HBcAb positive increasing the result to 34.1% positive HBcAb at the end of the study. Comparing this to the previous mentioned study in Palestine, the prevalence of anti-HBc positive cases was 31.8% pointing that the results for this test are comparable[12]. Comparing our results with nearby countries, the prevalence is lower in some neighbours like southern Greece (20.4%), but in others like Turkey (2.7%), central Greece (0.9%) and Iran (4.9%) our results were higher, same for further more developed countries like central Canada (3.8%) [17-24].
The association between HBcAb positivity at base line and HBcAb positivity after 6 months was significant at p<0.000 using Chi Square tests. Since anti-HBc positivity indicates previous exposure to HBV infection, such finding might suggest contact with HBV.
Furthermore, such data reflect a high HBV endemicity in our study hemodialysis patients as well as in those in other countries like Morocco, Iran, Turkey and Greece. Even that there is no significant association between positive anti HBc and PCR results at baseline nor after 6 months by Chi square tests ,there is a significant association (p<0.01) between positive anti-HBc and Occult hepatitis B (defined as positive anti HBc and positive HBV DNA PCR) after 6 months using Chi square tests, the thing that could mean that the results of anti-HBc and HBV DNA PCR are comparable suggesting a possibility of using anti-HBc instead of PCR for diagnosis of Occult Hepatitis B infection in hemodialysis patients. This suggestion is not novel, as many studies showed that detection of anti-HBc alone could reflect occult HBV infection in high-risk groups in areas where the HBV prevalence is high like Iran, Ghana, Oman and Brazil [24-28]. However, this needs further investigation by more studies.
D- Anti-HBc and hemodialysis session
The association between the hemodialysis sessions at baseline with HBcAb test was not significant using Chi square tests. Interestingly, the result was different after 6 months; there was significant association between being HBcAb positive and hemodialysis session (p=0.013). Moreover, similar findings were reported by dividing the hemodialysis sessions into two groups according to days (Saturday-Monday-Wednesday vs. Sunday-Tuesday- Thursday) (P<0.01 using Chi square tests). These findings raise the concern of possible incomplete adherence to standard and dialysis-specific infection control precautions leading to possible patient-to-patient any form of viral transmission that has taken place in NNHU hemodialysis unit.
Our study didn’t involve thorough investigations to assess the adherence of NNUH medical staff to infection control precautions (for example, using items taken to a patient’s dialysis station only for that patient, medications should be prepared in a room or area separated from the patient treatment area and designated only for medications, medications should be delivered separately to each patient, using clean trays not common carts and not puncturing IV medication vials labeled for single use more than once … etc.) (CDC). For example, several studies have showed Hepatitis B viral transmission possibly via multi-dose vials contaminated with blood from infected patient [29] .
Conclusion
Even though Hepatitis B is a common infection that affects millions of people worldwide every year, many cases of Occult Hepatitis B are missed especially in high prevalence areas among high risk groups. Transmission of such cases remains poorly understood and there is a significant gap in literature to be filled regarding the prevalence of Occult Hepatitis B infections in different sittings and the best ways to detect them thereby taking the necessary precautions to stop further spread. This study showed that Occult Hepatitis B infection was quite common among hemodialysis patients in NNUH emphasizing the existence of this problem in Palestine like other nearby countries. Moreover, this study joins many previous others declaring that detection of anti-HBc alone could reflect occult HBV infection in high-risk groups in areas where the HBV prevalence is high. Finally this study elaborates the importance of complete adherence to standard and dialysis-specific infection control protocols and investigating any possible breaks in such precautions.
Limitations
We only explored the situation of HBV DNA in anti-HBc cases and not all cases; while, OHB infection can be detected in anti-HBc negative cases as well. However, many studies showed that detection of anti-HBc alone could reflect occult HBV infection in high-risk groups in areas where the HBV prevalence is high [24] .
Recommendations
- Performing a longitudinal follow up study to all anti-HBc positive patients to trance changes including seroconversion to positive HBV DNA fulfilling the definition of Occult Hepatitis B in addition to other possible long term complications ( liver cirrhosis, hepatocellular carcinoma … etc.).
- Revising the complete adherence to standard and dialysis-specific infection control precautions by appropriate assessment tools, infection control workshops and educational programs to patients, contacts and medical staff to limit the transmission of any form of infection transmission in dialysis unit.
Recommendation for further research:-
- More investigations about the presence of Occult Hepatitis B infection in other hemodialysis centers in Palestine as well as among other high risk patients.
- Further research is needed to investigate the possibility of using the detection of HbcAb as a screening tool for Occult hepatitis B instead of PCR.
- Studies investigating the role of possible independent predictors of HBV DNA status in cases with isolated anti-HBc, especially Diabetes mellitus as many studies confirm such association (others may include higher age, higher age when dialysis started, illiterate, disabled and retired, visual disturbances and history of depression).
- More studies to determine routine blood donor screening for different Hepatits B serology tests (including anti-HBc status and HBV DNA) to investigate possible cost effective decrease in the risk of post-transfusion HBV infection.
References
- Adane and S. Getawa. The prevalence and associated factors of hepatitis B and C virus in hemodialysis patients in Africa: A systematic review and meta-analysis. PLoS ONE. 2021; 16(6). June. Public Library of Science. doi: 10.1371/journal.pone.0251570.
- Zbinden, et al. Prevalence of Occult Hepatitis B Virus Infection in Blood Donors with Negative ID-NAT in Switzerland. Transfusion Medicine and Hemotherapy. 2022; 49(6): 338-345. doi: 10.1159/000525480.
- Liu, Y. Zhang, M. Xu, X. Li, Z. Zhang. Distribution of hepatitis B virus genotypes and subgenotypes. Medicine. 2021; 100(50): e27941. doi: 10.1097/MD.0000000000027941.
- Saitta, T. Pollicino, G. Raimondo. Occult Hepatitis B Virus Infection: An Update. Viruses. 2022; 14(7): 1504. doi: 10.3390/v14071504.
- Sarowar, G. Hirode, H. L. A. Janssen, J. J. Feld. Controversies in Treating Chronic Hepatitis B Virus Infection: Discordant Serologic Results. Clin Liver Dis. 2021; 25(4): 805-816. doi: https://doi.org/10.1016/j.cld.2021.06.007.
- Razavi. Global Epidemiology of Viral Hepatitis. Gastroenterol Clin North Am. 2020; 49(2): 179-189. doi: https://doi.org/10.1016/j.gtc.2020.01.001.
- Palestine Health Status Annual Report 2016. Ministry of Health (Palestine). 2016.
- Soi and S. Soman.Preventing Hepatitis B in the Dialysis Unit. Advances in Chronic Kidney Disease. W.B. Saunders. 2019; 26(3): 179-184. doi: 10.1053/j.ackd.2019.03.003.
- Elamin and H. Abu-Aisha. Prevention of Hepatitis B Virus and Hepatitis C Virus Transmission in Hemodialysis Centers: Review of Current International Recommendations. Arab J Nephrol Transplant. 2011; 4(1). doi: 10.4314/ajnt.v4i1.63154.
- M. Ruiz-Calero Cendrero, et al. Cuándo puede ser útil buscar VHB oculto en pacientes en hemodiálisis?. Nefrología. 2020; 40(2): 115-119. doi: 10.1016/j.nefro.2019.07.001.
- Madihi, S. Madihi, H. Syed, F. Lazar, A. Zyad, A. Benani. A Systematic Review of the Current Hepatitis B Viral Infection and Hepatocellular Carcinoma Situation in Mediterranean Countries. BioMed Research International. 2020. Hindawi Limited. doi: 10.1155/2020/7027169.
- Dumaidi, A. Al-Jawabreh. Prevalence of occult HBV among hemodialysis patients in two districts in the northern part of the West Bank, Palestine. J Med Virol. 2014; 86(10): 1694-1699. doi: 10.1002/jmv.24008.
- M. Alzahrani, Muzaheed, S. S. Shaikh, A. I. Alomar, S. Acharya, N. Elhadi. Prevalence of Hepatitis B Virus (HBV) among blood donors in eastern Saudi Arabia: Results from a five-year retrospective study of HBV seromarkers. Ann Lab Med. 2018; 39(1): 81-85. doi: 10.3343/alm.2019.39.1.81.
- N. A. Said. An overview of occult hepatitis B virus infection. World J Gastroenterol. 2011; 17(15): 1927. doi: 10.3748/wjg.v17.i15.1927.
- Raimondo, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008; 49(4): 652-657. doi: 10.1016/j.jhep.2008.07.014.
- H. Gerlich, et al. Occult Hepatitis B Virus Infection: Detection and Significance. Digestive Diseases. 2010; 28(1): 116-125. doi: 10.1159/000282074.
- Y. Minuk, et al. Occult hepatitis B virus infection in a North American adult hemodialysis patient population,” Hepatology. 2004; 40(5): 1072-1077. doi: 10.1002/hep.20435.
- Siagris, et al. Occult hepatitis B virus infection in hemodialysis patients with chronic HCV infection. J Nephrol. 2006; 19(3): 327-33.
- Goral. Prevalence of occult HBV infection in haemodialysis patients with chronic HCV. World J Gastroenterol. 2006; 12(21) 3420. doi: 10.3748/wjg.v12.i21.3420.
- Yakaryilmaz, et al. Prevalence of Occult Hepatitis B and Hepatitis C Virus Infections in Turkish Hemodialysis Patients. Ren Fail. 2006; 28(8): 729-735. doi: 10.1080/08860220600925602.
- Mina. Prevalence of occult hepatitis B virus infection in haemodialysis patients from central Greece. World J Gastroenterol. 2010; 16(2): 225. doi: 10.3748/wjg.v16.i2.225.
- Tramuto, C. M. Maida, G. M. E. Colomba, P. Di Carlo, F. Vitale. Prevalence of occult hepatitis B virus infection in a cohort of HIV-positive patients resident in Sicily, Italy. Biomed Res Int. 2013; 2013: 859583. doi: 10.1155/2013/859583.
- Joukar, F. Mansour-Ghanaei, S. Besharati, and M. Khosh-Sorur. Occult hepatitis B infection in a hemodialysis population in Guilan province, northern Iran. Hemodialysis International. 2012; 16(2): 294-297. doi: 10.1111/j.1542-4758.2011.00645.x.
- Keyvani, S. Agah, A. Kabir, and S. M. Alavian, “Prevalence and risk factors of isolated anti-HBc antibody and occult hepatitis B infection in hemodialysis patients: a nationwide study. Ann Hepatol. 2013; 12(2): 213-9.
- Ramezani, M. Banifazl, A. Eslamifar, and A. Aghakhani. Serological pattern of anti-HBc alone infers occult hepatitis B virus infection in high-risk individuals in Iran,” The Journal of Infection in Developing Countries. 2010; 4(10): 658-661. doi: 10.3855/jidc.728.
- Allain JP, et al. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood. 2003; 101(6): 2419-2425. doi: 10.1182/blood-2002-04-1084.
- Stolf-Moreira, et al. Soybean physiology and gene expression during drought. Genetics and Molecular Research. 2010; 9(4): 1946-1956. doi: 10.4238/vol9-4gmr851.
- Kaminski, A. Alnaqdy, I. Al-Belushi, J. Nograles, S. H. Al-Dhahry. Evidence of Occult Hepatitis B Virus Infection among Omani Blood Donors: A Preliminary Study. Medical Principles and Practice. 2006; 15(5): 368-372. doi: 10.1159/000094271.
- Karanfilovska, R. Martin, H. Barton, K. S. Yap, and A. Cheng. Use of a radiopharmaceutical multidose dispenser for positron emission tomography: Risk assessment and mitigation measures for infection prevention. Infect Dis Health. 2020; 25(2): 101-106. doi: 10.1016/j.idh.2019.12.003.

