Case report - Volume 3 - Issue 4
Insulinoma in a thin, Lean Indian male
Md Ejaz Alam¹; Basharat Dar¹; Shoaib Pattu¹; Pooran Sharma¹; Abid Bhat¹; Saleem Baba¹; Shahnaz Mir²; Khursheed Bhat³; Mohammad Hayat Bhat⁴
¹Senior Resident, Department of Endocrinology, GMC Srinagar, India.
²Lecturer, Department of Endocrinology, GMC Srinagar India.
³Lecturer, Department of Endocrinology, GMC Srinagar India.
⁴Assistant professor, Department of Endocrinology, GMC Srinagar, India.
Received Date : July 17, 2023
Accepted Date : Aug 15, 2023
Published Date: Aug 24, 2023
Copyright: © Md Ejaz Alam 2023
*Corresponding Author : Md Ejaz Alam, Senior Resident, Department of Endocrinology, SSH, GMC, Srinagar, India.
Email: ejazpmch@gmail.com
DOI: Doi.org/10.55920/2771-019X/1532
Abstract
Insulinoma is a tumor that secretes insulin, leading to hypoglycemia as a result of excessive insulin release. The diagnosis of insulinoma is based on the presence of Whipple’s triad, which includes the following criteria: symptoms of hypoglycemia such as tremor, sweating, irritability, uneasiness, and weakness; a plasma glucose concentration of less than 55 mg/dL (3.0 mmol/L); and resolution of symptoms upon administration of glucose. A 30-year-old male presented with recurrent episodes of palpitations, sweating, dizziness, and vision difficulty, primarily occurring in a fasting state and relieved by food ingestion. Investigations revealed hypoglycemia during an episode, along with endogenous hyperinsulinism. Imaging studies showed a hyperenhancing lesion in the pancreatic body, which was confirmed as a neuroendocrine tumor on histology and immunohistochemistry. The patient underwent enucleation of the lesion, resulting in the normalization of plasma glucose levels postoperatively.
Keywords: Insulinoma, Hypoglycemia, Whipple’s triad, Endogenous hyperinsulinism, Neuroendocrine tumor
Introduction
Insulinoma is a rare type of functional neuroendocrine tumor (NET) characterized by a small insulin-secreting tumor that leads to excessive insulin secretion and subsequent hypoglycemia. Its incidence is exceptionally low, with an annual frequency ranging from 1 to 4 cases per million per year [1,2]. Here, we present the case of a 30-year-old male who presented with recurring episodes of palpitations, sweating, dizziness, and vision difficulties, primarily occurring during periods of fasting and alleviated by consuming food. These symptoms are typical manifestations of hypoglycemia induced by the excessive release of insulin from the insulinoma. The gold standard for diagnosing insulinoma biochemically involves the evaluation of plasma glucose, insulin, beta-hydroxybutyrate, and C-peptide levels during a 72-hour fasting test. This fasting test can effectively differentiate up to 99% of insulinomas from other conditions [3]. Surgical resection is considered the treatment of choice for insulinoma. Before the procedure, imaging studies, such as a computed tomography (CT) scan, are performed to locate the tumor accurately. The two most commonly employed surgical techniques for insulinoma are enucleation of the tumor and partial distal pancreatectomy [1].
Case Presentation
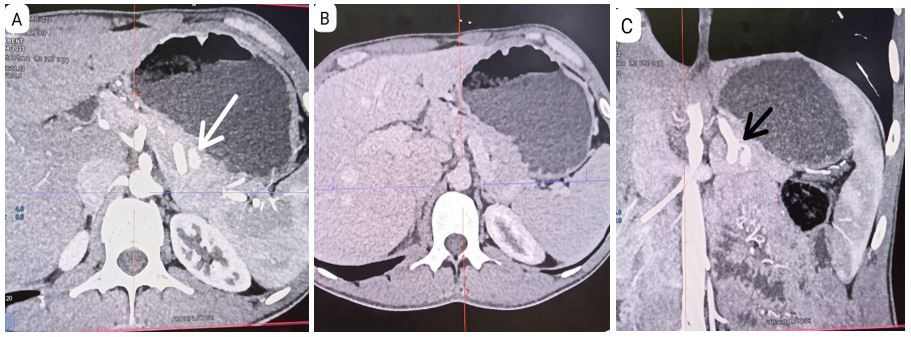
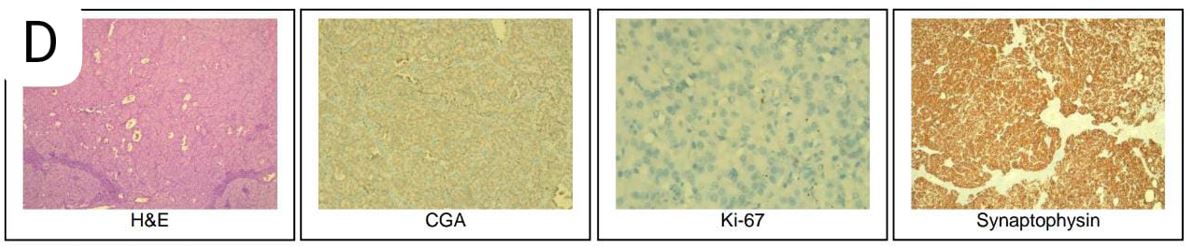
A 30-year-old male who presented with a 1.5-year history of recurrent episodes of palpitations, sweating, dizziness, and vision difficulty. The symptoms initially occurred 2-3 times per week and gradually increased to 8-10 episodes per week, and occurred in the morning after waking up in a fasting state, as well as occasionally in the evening while at work. The symptoms were not present in the post-meal phase or during the night. The patient experienced similar episodes daily during the first 20 days of Ramadan fasting, occurring at 5-6 pm before iftar. The patient also reported feeling an increase in hunger during these episodes. He had no history of alcohol or drug intake, fever, jaundice, loss of appetite, leg edema, periorbital puffiness, nausea, vomiting, fainting, fall, increased pigmentation of the skin on sun-exposed areas, or increased craving for salty food. On further examination, the patient's fasting blood glucose levels were found to be low, with values of 51, 45, and 41 mg/dl. The patient's father was recently diagnosed with type 2 diabetes. The patient has no significant medical history and does not smoke or use tobacco. On physical examination, the patient's vital signs were within normal limits. Anthropometry measurements revealed a BMI of 18.9. General examinations was normal. The diagnosis of multiple endocrine neoplasia type 1 (MEN1) was excluded based on history, physical examination, and laboratory Investigations (i.e., no history of dyspepsia, or other symptoms of GI ulcers, bone pain or renal stones). Serum calcium was 10.1 mg/dL. The patient's HbA1c was 4.3%. However, his blood glucose level was found to be very low (31mg/dl) during the critical care sample, which is concerning. Anterior pituitary hormone was normal. The insulin level was 8.1 mIU/L and C-peptide level of 1.86 ng/mL, as well as a suppressed Beta hydroxybutyrate level of 0.15 mmol/L. The ultrasound of the pancreas did not reveal any abnormalities, however, the triple phase contrast CT scan with showed a arterial phase 12×7mm hyperenhancing lesion in pancreatic body (Panel A) showing washout becoming isoattenuating to parenchyma ( Panel B), lesion has arterial feeding artery from splenic artery (Panel C) but without liver metastasis or intra-abdominal lymph nodes. Subsequently, the patient underwent surgery, specifically enucleation. Histology & IHC Features are consistent with Neuroendocrine tumour, Well differentiated (G1) with Mitotic Rate: < 2 mitoses / 2 mm², Ki-67 Labelling Index: < 3%. Immunoreactive IHC marker: Synaptophysin and CGA - score 4+ in neoplastic cells, Ki-67-stains 2-3% of neoplastic cells. (Panel D). Postoperative ultrasound of the abdomen did not show any intra abdominal collection, and amylase levels did not indicate the presence of postoperative pancreatic fistula or pancreatitis. The patient's blood glucose levels were maintained within the range of 110-150 mg/dl.
Discussion
An insulinoma is a neuroendocrine tumor characterized by excessive production of insulin, leading to hypoglycemia. The majority of insulinomas are benign and solitary. Based on estimations, around 10% of insulinomas exhibit multiplicity, meaning they manifest in multiple locations within the body. Similarly, approximately 10% of these insulinomas are linked to multiple endocrine neoplasia type 1 (MEN1) syndrome. Furthermore, another 10% of insulinomas are classified as malignant, indicating their cancerous nature. Lastly, about 10% of insulinomas are considered ectopic, indicating their unusual location outside the pancreas.
The signs and symptoms of an insulinoma include fasting hypoglycemia, which presents with autonomic symptoms referred to as “sympathoadrenal symptoms,” as well as episodes of neuroglycopenia. In some cases, postprandial hypoglycemia may be the only manifestation [5]. In individuals with insulinoma, hypoglycemia is primarily caused by decreased hepatic glucose output rather than increased glucose use [6]. Insulinomas originate from the islet cells of the pancreas, rather than from the ductular/acinar system [7].
Figure 1: Triple phase contrast CT scan showed arterial phase hyperenhancing lesion (12×7mm) in pancreatic body (Panel A) with complete washout (Panel B) and splenic artery as feeder vessel (Panel C). Histology & IHC Features are consistent with Neuroendocrine tumour, Well differentiated (G1) with Mitotic Rate: < 2 mitoses / 2 mm², Ki-67 Labelling Index: < 3%. Immunoreactive IHC marker: Synaptophysin and CGA – score 4+ in neoplastic cells, Ki-67-stains 2-3% of neoplastic cells. (Panel D).
The diagnosis of insulinoma is based on the presence of Whipple’s triad, which includes symptoms of neuroglycopenia (such as behavioral changes, confusion, and irritability) and sympathoadrenal symptoms (such as palpitations and tremors) in conjunction with low serum glucose levels (<50 mg/dL). These symptoms typically resolve after the administration of glucose [8]. The prevalence of Insulinoma is reported to be approximately 1 to 4 cases per million people per year [1,9]. Neuroglycopenic symptoms of insulinoma include agitation, disorientation, and changes in vision and behavior, while sympathoadrenal symptoms may manifest as palpitations, sweating, and tremulousness [5,8]. Weight gain has been observed in 18% of patients [10]. In general, symptoms of hypoglycemia occur primarily in the fasting state in about 73.5% of patients, while 21% report experiencing symptoms both during fasting and after meals, and 6% exhibit symptoms exclusively after meals [5].
A variety of techniques are employed to localize an insulinoma, including transhepatic portal venous sampling, arteriography, transabdominal ultrasonography, intraoperative ultrasonography, abdominal computed tomography (CT) scan, magnetic resonance imaging (MRI), endoscopic ultrasonography (EUS), intraoperative palpation, selective arterial calcium stimulation with hepatic venous sampling, and 111 In-labeled octreotide scans with single-photon emission tomography. The reported sensitivity of some of these methods for identifying pancreatic insulinomas is as follows: MRI (37-71%), CT scan (30-66%), EUS (37-71%). When MRI, CT scan, and EUS are combined, the reported sensitivity for insulinoma identification ranges from 17% to 66%. Arteriography was previously considered the gold standard for insulinoma localization, but its use has been limited due to documented sensitivities of 29-64% and the availability of superior noninvasive imaging techniques [11]. Overall, the long-term survival rate for individuals with insulinoma is high, and it is estimated that approximately 90-95% of insulinomas exhibit benign histological behavior. Therefore, complete removal of the tumor typically leads to resolution of symptoms [12]. In a series of 120 patients between the ages of 4 and 17 years, only 5.4% of cases experienced recurrences, which were subsequently managed through surgical intervention [13].
Conclusion
Insulinoma is a rare tumor that can occur sporadically and is not typically associated with obesity. In fact, most patients with insulinoma do not exhibit hyperphagia or obesity. Instead, they often experience repeated episodes of hypoglycemia, which can be relieved by consuming meals. Therefore, healthcare providers should maintain a high index of suspicion when evaluating patients who present with recurrent hypoglycemic events that resolve with food intake. Additionally, it is important to note that the patient in the given case is lean, with a BMI of 18.9, which further supports the atypical presentation of insulinoma in this individual.
References
- Functioning insulinoma—incidence, recurrence, and long-term survival of patients: a 60-year study. Service FJ, McMahon MM, O’Brien PC, Ballard DJ. Mayo Clin Proc. 1991; 66: 711-719.
- ENETS 2011 Consensus Guidelines for the management of patients with digestive neuroendocrine tumors: an update. Salazar R, Wiedenmann B, Rindi G, Ruszniewski P. Neuroendocrinology. 2012; 95: 71-73.
- The prolonged fast. Service FJ, Natt N. J Clin Endocrinol Metab. 2000; 85: 3973-3974.
- Pancreatic insulinoma. Case report and review of the literature. Negrean V, Tudor A, Aioanei O, Domsa I.
- Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. Placzkowski KA, Vella A, Thompson GB, et al. J Clin Endocrinol Metab. 2009; 94: 1069-1073.
- Pathogenesis of hypoglycemia in insulinoma patients: suppression of hepatic glucose production by insulin. Rizza RA, Haymond MW, Verdonk CA, Mandarino LJ, Miles JM, Service FJ, Gerich JE. Diabetes. 1981; 30: 377-381.
- Non-islet origin of pancreatic islet cell tumors. Vortmeyer AO, Huang S, Lubensky I, Zhuang Z. J Clin Endocrinol Metab. 2004; 89: 1934-1938.
- Insulinoma: clinical and diagnostic features of 60 consecutive cases. Service FJ, Dale AJ, Elveback LR, Jiang NS.
- ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Jensen RT, Cadiot G, Brandi ML, et al.
- Neuroglycopenic and other symptoms in patients with insulinomas. Dizon AM, Kowalyk S, Hoogwerf BJ. Am J Med. 1999; 106: 307-310.
- Hypoglycemic disorders. Service FJ. N Engl J Med. 1995; 332: 1144-1152.
- Ali ZA. Insulinoma. https://emedicine.medscape.com/article/283039-overview 2013
- Siperstein A, Clark OH, Rushakoff RJ. Endotext. 2013. Chapter 39: Insulinoma and other hypoglycemias.