Case report - Volume 3 - Issue 4
Eruptive Sebaceous Hyperplasia: A rare adverse effect of systemic corticosteroid use in two immunosuppressed patients
Casper Poulsen1*; Kristian Bakke Arvesen2; Luit Penninga3; Peter Bjerring4; Carsten Sauer Mikkelsen5
1Research Unit, Department of Dermatology, Aalborg University Hospital, Aalborg, Denmark.
2Specialist in Dermatology, Research Unit, Department of Dermatology, Aalborg University Hospital, Aalborg, Denmark.
3Specialist in Surgery, Ph.D., Department of Surgery and Transplantation, Rigshospitalet, Copenhagen, Denmark.
4Specialist and professor of Dermatology, Department of Dermatology, Aalborg University Hospital, Denmark.
5Specialist in Dermatology, Private Dermatology Clinic, Brønderslev and Senior Research Fellow, Research Unit, Department of Dermatology, Aalborg University Hospital, Aalborg, Denmark.
Received Date : July 19, 2023
Accepted Date : Aug 22, 2023
Published Date: Aug 29, 2023
Copyright: © Casper Poulsen 2023
*Corresponding Author : Casper Poulsen, Research Unit, Department of Dermatology, Aalborg University Hospital, Aalborg, Denmark.
Email: CASPPU@rm.dk
DOI: Doi.org/10.55920/2771-019X/1535
Introduction
Sebaceous hyperplasia is a benign, not uncommon cutaneous disorder. The disorder is caused by overabundance and proliferation of sebocytes in the sebaceous glands [1]. The proliferation induces enlargement of the sebaceous gland up to several times its regular size, but still keeping the normal anatomical structures. Sebacceous glands have a central duct that may become dilated in sebaceous hyperplasia due to secretion of an oily substance, sebum, which presents flesh- or yellowish coloured [2]. Sebaceous glands are localized everywhere on the skin except for palms and soles of the feet. The highest concentration of the glands is found on the face, the back, and the upper arms, which is also the most common places to encounter sebaceous hyperplasia [3]. Papules that are newly formed often swell during sweating which is pathognomonic for the disorder. Sebaceous glands are commonly associated with hair follicles, though may rarely present in hairless regions of the skin [4].
The pathogenesis of sebaceous hyperplasia is probably related to the fact that sebaceous glands are highly sensitive to androgen levels in the blood. A decrease in hormonal androgen levels which occur for men around their eighth decade and for women around the menopause stimulates sebocyte proliferation and causes sebaceous hyperplasia. Due to a higher difference in circulating androgens the disorder primarily affects men [2]. Eruptive sebaceous hyperplasia can occur as an inherited disorder, which is called familial eruptive sebaceous hyperplasia [5]. Sebaceous hyperplasia is often reported in patients who are treated with long-term immunosuppressive agents following solid organ transplantation, especially cyclosporine. Cyclosporine has specifically been linked to the stimulation of sebaceous gland proliferation,and 10-16% of patients treated with cyclosporine develop eruptive sebaceous hyperplasia [6]. Approximately 1% of the healthy population is affected by sebaceous hyperplasia [2].
There have only been few reports on the occurrence of eruptive sebaceous hyperplasia associated with immunosuppression with prednisolone [7-8]. In the present two cases, we also report sebaceous hyperplasia associated with systemic corticosteroid use. Patients often are treated for cosmetic reasons, particularly because the disorder primarily affects the face. Eruptive sebaceous hyperplasia can be successfully treated with isotretinoin as well as with CO2 laser treatment [5, 9]. Eruptive sebaceous hyperplasia is a benign cutaneous disorder. Especially organ transplant recipients receiving immunosuppressive agents as cyclosporine, are at high risk of developing eruptive sebaceous hyperplasia, but also other skin diseases including non-melanoma skin cancers. Here, we present two cases of eruptive sebaceous hyperplasia in patients with long-term systemic corticosteroid treatment.
Case 1:
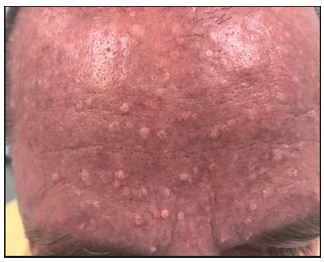
A 49-year-old Caucasian man treated with a renal transplant 25 years ago presented with a sudden appearance of multiple small yellow bumps in the face, primarily on the forehead. Since the patient had a renal transplant, he had been on longterm systemic treatment with prednisolone, and short-term treatment with cyclosporin and azathioprine. Skin examination by a dermatologist revealed multiple 1–5 mm, soft, skincolored to yellow, dome-shaped papules primary on the forehead but also bilaterally on the cheeks, clinically suggestive and confirmed histologically as sebaceous hyperplasia (Figure 1). He was treated with topical retinoid and CO2 laser treatment, 0.8 Watt, defocused beam on the affected areas with good effect.
Figure 1: (photo: Carsten Sauer Mikkelsen): Multiple umbilicated papules on the forehead of a patient 25 years after renal transplantation.
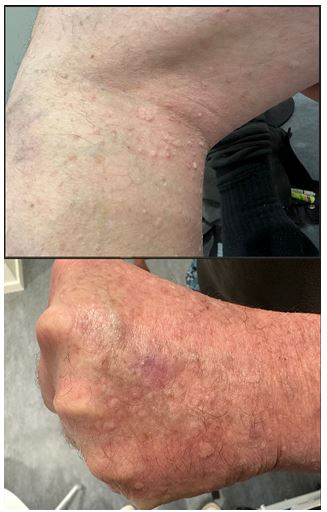
Figure 2 & 3: (photos: Carsten Sauer Mikkelsen): Multiple umbilicated papules in the poplitea-region and on the dorsal part of his hands.
Case 2:
A 50-year-old Caucasian man known with ulcerative colitis received long-term treatment with both prednisolone and azathioprine. The patient was consulted by a dermatologist due to eruption of multiple asymptomatic skin-colored to slight yellow, umbilicated elevated papules on the dorsal side on the hands bilaterally as well as bilaterally in the popliteal fossae. The papules varied in size from 1-3 mm. The patient was successfully treated for his condition with CO2 laser.
Conclusion
Eruptive sebaceous hyperplasia is a well-described dermatological disorder. It is common in immunosuppressed solid organ transplant recipients treated with cyclosporine. To our knowledge, this is only the second and third reported cases of eruptive sebaceous hyperplasia secondary to the use of systemic prednisolone.
References
- Hussein L, Perrett CM. Treatment of sebaceous gland hyperplasia: a review of the literature. J Dermatolog Treat. 2021 Dec;32(8):866-877. doi: 10.1080/09546634.2020.1720582. Epub 2020 Apr 13. PMID: 32011918.
- Farci F, Rapini RP. Sebaceous Hyperplasia. 2022 Sep 5. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 32965819.
- de Berker DA, Taylor AE, Quinn AG, Simpson NB. Sebaceous hyperplasia in organ transplant recipients: shared aspects of hyperplastic and dysplastic processes? J Am Acad Dermatol. 1996 Nov;35(5 Pt 1):696-9. doi: 10.1016/s0190-9622(96)90723-9. PMID: 8912563.
- James WD, Berger TG, Elson D. Andrews’ Diseases of the Skin: Clinical Dermatology. Saunders Elsevier. 2006, p. 662.
- Grimalt R, Ferrando J, Mascaro JM. Premature familial sebaceous hyperplasia: successful response to oral isotretinoin in three pa-tients. J Am Acad Dermatol 1997; 37: 996–998.
- Boschnakow A, May T, Assaf C, Tebbe B, Zouboulis ChC. Ciclosporin A-induced sebaceous gland hyperplasia. Br J Dermatol. 2003 Jul;149(1):198-200. doi: 10.1046/j.1365-2133.2003.05397.x. PMID: 12890221.
- Mikkelsen CS, Penningua L, Abusland TB et al. Eruptive Sebaceous Hyperplasia: An Uncommon Side Effect of Systemic Corticosteroid Use in a Renal Transplant Patient. Forum for Nord Derm Ven 2019, Vol. 24, No. 1
- Ranasinghe GC, Friedman AJ. Eruptive Sebaceous Hyperplasia: A Rare Consequence of Systemic Corticosteroids. J Drugs Dermatol. 2018 Jan 1;17(1):118-120. PMID: 29320598.
- Levandoski KA, Girardi NA, Loss MJ. Eruptive sebaceous hyperpla-sia as a side effect of oral tacrolimus in a renal transplant recipient. Dermatol Online J 2017 23. pii: 13030/qt7x0125gz.