Research Article - Volume 3 - Issue 5
Risk factors for anti-infliximab antibody formation among patients with inflammatory bowel disease
Abdoh Qusay1,4*; Alnees Mohammed1; Rabi R1; Kittaneh Ameer1; Dweikat A1; Darwish Abdalaziz1; Najajra Duha1; Mahdi Awwad1; Yaghmour Raghad1
1Department of Medicine, Faculty of Medicine & Health Sciences, An-Najah National University, Nablus, Palestine.
2Department of Internal Medicine,GI and Endoscopy unit , An-Najah National University Hospital, Nablus, Palestine.
Received Date : July 20, 2023
Accepted Date : Aug 30, 2023
Published Date: Sep 06, 2023
Copyright: © Abdoh Qusay 2023
*Corresponding Author : Abdoh Qusay, Department of Medicine, Faculty of Medicine & Health Sciences, An-Najah National University, Nablus, Palestine.
Email: qusayabdoh@najah.edu
DOI: Doi.org/10.55920/2771-019X/1541
Abstract
Background: Infliximab (IFX) is an effective therapy for Inflammatory Bowel Disease (IBD), but it may be associated with a high rate of primary and secondary failure. Approximately 30% of patients lose their response. One loss of response to Anti-Drug Antibody (ADA) development is the most common cause of this loss.
Objective: This study aims to demonstrate the incidence of antibody formation against infliximab when used to treat Inflammatory Bowel Disease (IBD) and risk factors that may be associated with its formation, to find if there are correlations between antibody formation and patient characteristics, other co-medication, infliximab level, doses, duration, etc., and the subsequent correlation of antibody formation to Primary nonresponse, Secondary failure, Maintained response, and finally, to assess the overall clinical impact on the patients.
Methods: A retrospective study included 61 participants who were treated with Infliximab (IFX) for inflammatory bowel disease and received follow-up at. Patients were recruited, and relevant demographic, clinical, and laboratory data were recorded from patients’ files, themselves, or the patient's family. as the antibody level was drawn for all patients, and therefore those with positive antibodies who met our criteria entered the exposure group, while the negative patients were in the non-exposure group.
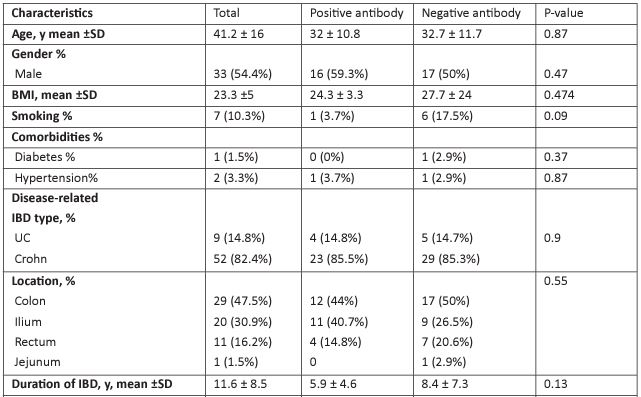
Results: There were 61 patients with IBD under infliximab therapy; 82% had Crohn's disease, and the rest had ulcerative colitis. Patient factors significantly associated with lower maintenance infliximab doses include age, smoking, aminosalicylates, and secondary non-response. Those of younger ages were more likely to develop infliximab-anti-drug antibodies (IFX-ADA) (OR, 95% CI; 1.12,1.007-1.25), and those who needed the use of amino-salicylates showed more tendency to develop IFX-ADA (OR, 95% CI; 0.03,0.01-0.74), AS 11.8% of participants with negative antibodies had used amino-salicylates compared to the other group, in which 3.7% of them used this. Higher doses (342.3 ± 94.54 mg) helped to obtain a better outcome by reducing the prevalence of ADA formation.
Conclusions: IFX-ADA is significantly influenced by maintenance infliximab dose, age, smoking, aminosalicylates, and secondary non-response. There should be a decreasing IFX dose interval strategy. Aminosalicylates may help reduce the formation of IFX-ADA.
Keywords: Risk factors inflammatory bowel disease anti-infliximab antibody.
Abbreviations
IBD: Inflammatory Bowel Disease; TNF: Tissue Necrosis Factor; IFX: Infliximab; ADA: Anti-Drug Antibodies; INF-ADA: Infliximab- Anti-Drug Antibodies; LOR: Loss Of Response; Abs: Antibodies; CRP: C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate; IRB: Institutional Review Board; Mo: Month; Y: Year; TC: Trough Concentration.
Key messages of your article: Infliximab (IFX) is an effective therapy for Inflammatory Bowel Disease (IBD), but it may be associated with a high rate of primary and secondary failure. This study aims to demonstrate the incidence of antibody formation against infliximab when used to treat Inflammatory Bowel Disease (IBD) and the risk factors that may be associated with its formation to assess the overall clinical impact on patients. There should be a decreasing IFX dose interval strategy and Higher starting doses that may improve outcomes for IFX-treated patients with Crohn's disease and ulcerative colitis. Aminosalicylates may help reduce the formation of IFX-ADA.
Background
Biologic therapy has evolved in recent years as the mainstay of treatment in Inflammatory Bowel Disease (IBD). Despite the cause of this condition being yet unknown, it is considered a result of an inappropriate inflammatory response, in which Tissue Necrosis Factor (TNF) is a central mediator. Thus, anti-TNF drugs like Infliximab (IFX) improved moderate-to-severe IBD [1]. Despite this success, they have limitations, including a high rate of primary and secondary failure. Approximately 30% of patients do not respond. There are several explanations for this loss of response; however, Anti-Drug Antibody (ADA) development is the most common cause of this loss [2]. Production of Infliximab-Anti-Drug Antibodies (INF-ADA) results from recognition of IFX by immunity, which targets TNF-binding sites of IFX and prevents IFX from binding to TNF; this, therefore, causes the elimination of drug and treatment failure and the cessation of IFX [3]. Immunogenicity against biological therapies was demonstrated in many ways. For example, higher rates of remission and fewer clinical benefits of the drug Moreover, reduced drug concentrations in the plasma In this situation, giving biological therapies in combination with concomitant immunosuppressive agents has been shown in several studies to reduce the development of antibodies. Switching to another biological therapy or using it in combination also showed a better response [4]. Studies showed both drug level and antibody formation are mainly used to decide treatment based on clinical activity [5]. In the Dotan et al. study, 54 Inflammatory Bowel Disease (IBD) patients were followed prospectively to assess the level of infliximab and antibody levels. They found infliximab clearance (shorter half-life) was significantly higher in those with antibodies (p 0.001). comparing those with positive antibodies with negative ones, had more infusion reactions, were previous smokers, used steroids, and had increased disease activity by Mayo score (p-value =0.023, 0.023, 0.0136, and 0.0017, respectively) [2]. In the PANTs study, we observed prospectively IBD patients who are getting Infliximab (IFX) and Adalimumab (ADM) as a treatment to find the relationship between getting the antibody against the drugs and the HLA-DQA1*05 allele. The results showed us that carriage of one or more HLA-DQA1*05 alleles develops a 2-fold risk of immunogenicity [6]. Infliximab (IFX) is an anti-TNF agent, which is one of the most effective IBD therapies currently known. Unfortunately, this couldn't be true for a subset of IBD patients who experience Loss Of Response (LOR), which may occur in 50% of patients, especially in the first year. The main mechanism leading to this LOR is antibody formation against infliximab, which happens in a range of 6.1 to 73% [1]. LOR results in a lack of clinical improvement after induction, so the medication will be stopped, subsequently resulting in therapy failure. Ultimately, this will require the switch to other therapies, all of which are cost-ineffective, time-consuming, and disappointing to both patients and their physicians for this reason. Therefore, this study was designed to assess the possible risk factors of getting antibodies against IFX when used to treat inflammatory bowel disease (IBD), to find if there is any correlation between getting the Antibodies (Abs) and the number of relapses, and finally, to determine if all of these had a clinical impact on the patients, as in the future we can anticipate the risk of these antibody formations before they occur to help guide therapy and optimize dosing based on specific patient factors, as it would be individualized therapy.
Material and methods
Study design and setting
A retrospective design was used as this is the best design to achieve our outcome. This study was carried out at as it provides continuous follow-up for a large number of patients with IBD. This study included all patients who were treated with Infliximab (IFX) for inflammatory bowel disease and received follow-ups at NNUH. The sample size was calculated considering a two-sided significance level of 5% and 80% power. Inclusion criteria were patients with IBD treated by IFX, compliance with the treatment, and continuous vetting of the follow-up. While exclusion criteria were: Getting IFX as a treatment for a disease that is not IBD; not complying with the treatment; and not getting a continuous follow-up.
A data collection sheet was constructed to collect data either from patients’ files, himself, or the patient's family. All patients who agreed to participate in the study were asked to complete the data collection sheet. Both cases and controls were chosen from patients attending gastroenterology clinics at the An-Najah National University Hospital (NNUH), as the antibody level was drawn for all patients, and therefore those with positive antibodies who met our criteria entered the case group, while the negative patients were in the control group. With a case-to-control ratio of 2 to 1, no matching was performed as we wanted to assess all risk factors between groups. The same data was collected from both cases and controls at the beginning of the study, retrospectively from the data record and patient interviews. Ethical approval was obtained from the Institutional Review Board (IRB) at An-Najah National University. Informed consent was requested from participants during the first clinical visit, and the participants were informed that their participation in the study was voluntary and that they could withdraw at any time without declaring the reason. Privacy and confidentiality were ensured.
Variables and definition
Infliximab antibody titers and duration since the beginning of treatment Independent variables: demographic data (age, sex, BMI), comorbidity, state of the smoker Disease status for Inflammatory Bowel Disease (IBD) (remission, mild, moderate-severe) and duration of the disease, location, used medications (glucocorticoid, budesonide, azathioprine, methotrexate, aminosalicylates), Laboratory tests: C-reactive protein (CRP), Erythrocyte Sedimentation Rate (ESR), albumin, calprotectin, and treatment outcome: Primary non-response: The complete absence of effect followed by discontinuation of therapy, Secondary failure: Initial response to infliximab induction and maintenance therapy, then followed by loss of effect with the return of active disease symptoms despite optimisation of dose and thus discontinuation of therapy, Maintained response: Sustained effect of infliximab effect on maintenance therapy during follow-up or at time of discontinuation, as well as Infliximab dose and intervals of administration.
Statistical analysis
Data were expressed as tables and graphs; comparisons between cases and controls were expressed as mean or median and tested by the appropriate significance tests for the study’s endpoints; Antibody risk factors were compared between Infliximab-Anti-Drug Antibodies (IFX-ADA) positive and IFX-ADA negative participants. Investigating the association of these main factors independently from each other and after adjusting for possible confounders Confounders were tested and adjusted for using binary logistic regressions. Moreover, subgroup analysis was performed comparing Inflammatory Bowel Disease (IBD). IBM SPSS statistical software version 25 was used for data entry and testing. The significance level was established at p 0.05.
Results
Of a total of 61 patients with IBD under infliximab therapy, 82% had Crohn's disease, the rest had ulcerative colitis, their mean age was 41.2 ± 16, 54% were men, and the average duration of IBD was 11.6 ± 8.5. 45 had concurrent use of glucocorticoids (73.5%), 43 had azathioprine (70.6%), and 5 had aminosalicylates (8.8%). The incidence of primary non-response was 2.9%, while secondary non-response was 32.4% among all participants (Table 1).
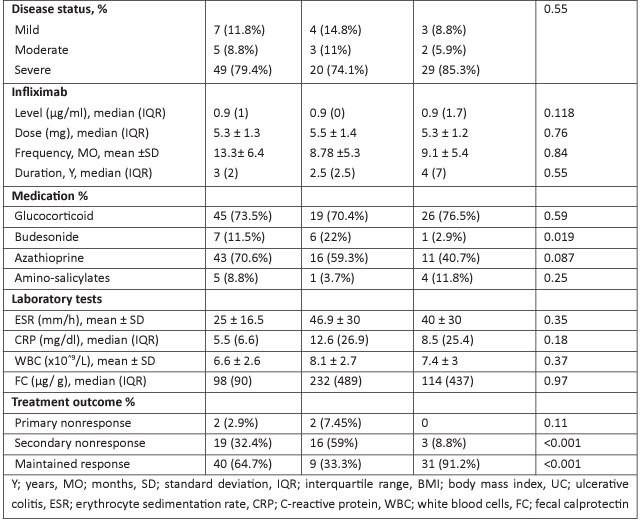
Of the 61 participants, 27 (44.3%) had positive infliximab antibody, the mean of the infliximab antibody mean was 1.13±1.5, comparing in terms of positive and negative the antibody (Table 1), infliximab level and frequency were very close, and a minor but insignificant difference in the duration of infliximab intake (median (IQR);4 (7) vs 2.5 (2.5) and its frequency between the two groups (mean± SD; 8.78 ±5.3 vs 9.1 ± 5.4).
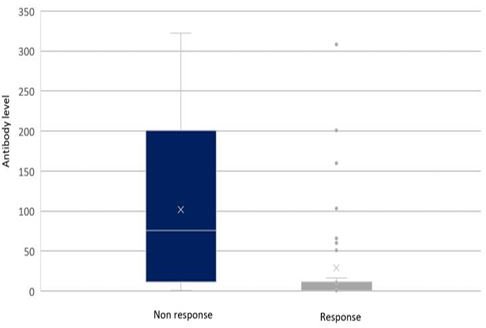
For the outcome of the disease, the majority of those who had a secondary non-response were positive for the infliximab antibody, yielding a significant p-value of 0.001 compared to those who had afavourable maintained response which was negative for the antibody (91%). Figure 1 showed the difference in levels of antibodies between those who had non-response and those who maintained response, with obviously higher levels of antibodies among those who developed non-response. The mean ± SD of antibody level was 101.7 96.5 vs. 29 67.6 among secondary non-response and maintained responses, respectively (Figure 1). No difference was observed in univariate analysis for demographics, comorbidities, concurrent medication, or lab tests.
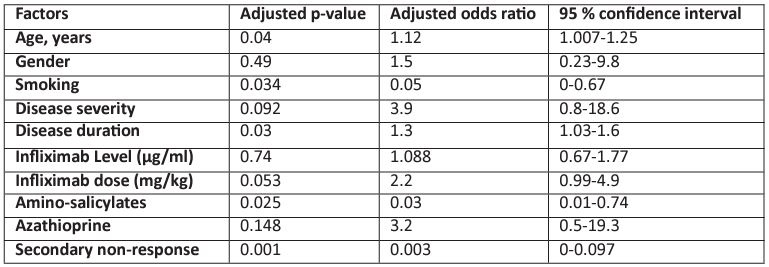
Regarding the dose of infliximab, most cases of Anti-Drug Antibody (ADA) formation were observed to be associated with a lower maintenance infliximab dose (326.5-70.3 mg) in our study. On the contrary, higher doses (342.3 ± 94.54 mg) helped to obtain a better outcome by reducing the prevalence of ADA formation (Figure 2). However, in the multivariate analysis (Table 2), a significant relationship was observed for age, smoking, aminosalicylates, and secondary non-response. Those of younger ages were more likely to develop infliximab-anti-drug antibodies (IFX-ADA) (OR, 95% CI: 1.12,1.007-1.25), and those who needed the use of amino-salicylates showed more tendency to develop IFX-ADA (OR, 95% CI: 0.03,0.01-0.74), AS 11.8% of participants with negative antibodies had used amino-salicylates compared to the other group, in which 3.7% of them used this Moreover, those who had IFX-positive ADA were significantly more likely to develop non-response, as shown in both univariate and multivariate analyses (non-response incidence was 59% vs. 8.8% for those who had positive IFX-ADA and negative antibodies, respectively), in which the OR, 95% CI, was 0.003, (0.05, 0-0.67). While those who smoke were less likely to have IFX antibodies (OR,95% CI; 0.003,0-0.097).
Discussion
The hindering obstacle against infliximab treatment, despite its great effectiveness, is the high incidence of response incidence,
Figure 1 Illustration of the difference in infliximab antibody levels between patients who had developed no response to therapy and those who had maintained the response
Table 1: Baseline characteristics and disease states with differences concerning anti-infliximab antibody.
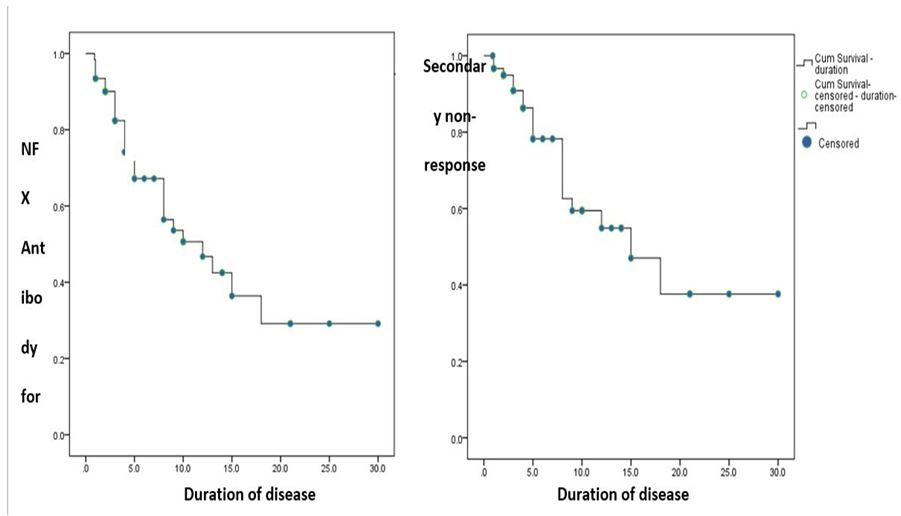
in which approximately 37% of initial responders had secondary losses in response to infliximab as shown in a meta-analysis [7], while approximately 20% loss of responsiveness per year in the study of Eun Hye Oh’ study [8]. Apparently, in our study, 31.8% had a loss of response to infliximab later in the course. Due to this, efforts to find the underlying factors for this loss of response had been made, and the proposed leaders in this process were the appearance of infliximab antibody appearance and the decrease in infliximab level as found in the literature [2, 9-11], and subsequent strategies to deal with this phenomenon were optimising and personalising the treatment algorithms based on drug and Anti-Drug Antibodies (ADA) levels [8]. These conclusions were confirmed in our study, in which the percentage of patients who had a loss of response to infliximab was significantly higher among ADA-positive patients than ADA-negative patients (85.7% vs. 14.3%, respectively, P-value <0.001). And it was observed that this loss of response was acquired primarily after 1.5 years; the same cutoff was also calculated for the appearance of the antibody appearance, supporting this strong relationship, especially noted for the antibody value of more than 4 U/ml, which is in the range of the previously identified threshold for loss of response or discontinuation of the drug, which ranged from 3 U/ml to 9 U/ml [12].
Figure 2: Infliximab antibody appearance in relation to disease duration, B; secondary occurrence in relation to disease duration.
Table 2: Factors associated with infliximab antibody formation.
Despite the limited number of participants, the prevalence of Anti-Drug Antibody (ADA) formation in our 42 patients was 38.6%, which is comparable to many reported numbers in other studies. 180 cases of IBD who received infliximab therapy in a single centre were studied for the formation of ADA during therapy to show that 46% of them were positive [9]. In Tournier et al.'s study, the incidence of infliximab antibody was 67% of patients [13]. The Bella Ungar observational study reported that the incidence of ADA formation is the same in the groups of patients with scheduled and episodic therapy, and it was 46% [14]. In addition, Tun et al.'s team followed up on 214 cases of IBD for 12 months in a cohort study to identify clinical ADA formation, and It revealed that 113 (53%) were positive. The apparent incidence of ADA was lower than in these studies [15].
On the other hand, another study did not show clinical significance between antibody appearance and lower infliximab level, which may mean that ADA may have another independent mechanism to cause loss of response than increasing drug clearance as hypothesised [7] and is similar to the finding of the Casteele et al. study, this still supports the hypothesis of neutralising the drug by blocking its actions [11], and another single study for infliximab clearance factors may be warranted. This strong relationship between the presence of ADA and loss of response highlights the importance of an ADA-based approach for the appropriate clinical strategies during maintenance therapy by identifying high-risk patients for the imminent loss of response LOR to infliximab, who usually, as our finding suggests, were antibody positive, after the introduction of a clinical approach, which can be of more cost and clinical effectiveness compared to the conventional dose intensification in those with a loss of response.
In terms of treatment methods, the PRECISION prospective trial compared dashboard-guided dosing with conventional dosing of infliximab in IBD treatment. Personalised infliximab dosing in IBD treatment showed a higher incidence of patients who had maintained remission during the study. And it is recommended to increase the infliximab target Trough Concentration (TC) in patients who failed to maintain remission due to perianal complications [16]. Another study demonstrated that 3–7 mcg/ml of TC of infliximab helped to achieve greater efficiency during treatment. Furthermore, >3.8 mcg/ml was considered adequate TC to have a predictive response [17].
In contrast to what we mentioned above for Bella Anger's trial results, where they shared that the incidence of ADA is the same in episodic and scheduled groups, ACCENT I and II showed a lower incidence of ADA formation in addition to a higher proportion of remission in scheduled group therapy, especially in the 10 mg/kg group over the 5 mg/kg group [18,19].
Though the addition of immunomodulators with biological therapy like infliximab has shown some effects in improving the efficacy of the biologic drug and suggested a strategy to prevent immunogenicity in some studies in IBD [8,20], it can also prevent ADA formation with even lower doses [3,21]. In this study, we did not detect any significant effects of immunomodulators (azathioprine or methotrexate) on the appearance of ADA, similar to Gomes et al. [22]. It is possible that this association can't be revealed since the majority of our sample was at low infliximab levels mean SD (1.5-2.1), as some studies found that patients with lower infliximab concentrations (especially lower than 5mg/mL) would have the same drug maintenance whether they had infliximab monotherapy or concomitant immunomodulators. A placebo-controlled clinical trial demonstrated that using 200 mg intravenous hydrocortisone immediately before the infusions of infliximab will reduce ADA formation by 26% compared with 42% in the placebo group [23].
The only demographic characteristic that we identified to be related to antibody formation was age, in which a higher rate of immunogenicity was observed among younger ages. In a study, younger people were more likely to develop a non-response to infliximab antibody (OR 0.961, 95% CI 0.926-0.998, p = 0.037), which indirectly correlates with our finding [4].
In this study, we aimed to explore the factors that may increase the risk of forming anti-infliximab antibodies in individuals with Inflammatory Bowel Disease (IBD). Our unique contribution lies in our focus on an often overlooked population group. We observed a total of 61 IBD patients who received infliximab therapy and were followed up accordingly. We discovered that certain patient characteristics, such as age and smoking status, and the use of aminosalicylates plays a significant role in the development of Infliximab Anti-Drug Antibodies (IFX ADA). Additionally, we found evidence suggesting that higher doses of infliximab can potentially reduce the prevalence of ADA formation. These findings emphasize the importance of tailoring treatment plans to each patient's specific needs, considering adjustments in dose intervals, and incorporating aminosalicylates to minimize antibody formation. Overall, our study sheds light on essential information regarding not only how frequently anti-infliximab antibodies occur but also the risk factors associated with their formation in an underrepresented population, thereby enhancing our understanding of treatment outcomes for IBD patients.
The limitations of this study were: The small sample size is the most important limitation of this study, as not all patients come for follow-up, and there is not enough awareness in our society of IBD, which makes the diagnosed population much smaller than the diseased population. In addition, adalimumab is a newly used generation at our hospital, so we rely only on infliximab in this study.
Conclusions and recommendations
Acknowledgment: The authors acknowledge all participants in this study for their cooperation and contribution.
Conflicts of interest: The authors declare that there are no competing interests.
Financial support: The authors have declared that they had no financial support related to this study.
Data availability statement: The data sets supporting the results of the current research are available from the corresponding authors upon request.
References
- Hindryckx P, Novak G, Vande Casteele N, Khanna R, Laukens D, et al. Incidence, Prevention, and Management of Anti-Drug Antibodies Against Therapeutic Antibodies in Inflammatory Bowel Disease: A Practical Overview. Drugs. 2017; 77: 363-77.
- Dotan I, Ron Y, Yanai H, Becker S, Fishman S, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis. 2014; 20: 2247-59.
- Grinman AB, de Souza MdGC, Bouskela E, Carvalho ATP, de Souza HSP. Clinical and laboratory markers associated with anti-TNF-alpha trough levels and anti-drug antibodies in patients with inflammatory bowel diseases. Medicine. 2020; 99: e19359.
- Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. Therap Adv Gastroenterol. 2018; 11.
- Reinhold I, Blümel S, Schreiner J, Boyman O, Bögeholz J, et al. Clinical Relevance of Anti-TNF Antibody Trough Levels and Anti-Drug Antibodies in Treating Inflammatory Bowel Disease Patients. Inflammatory Intestinal Diseases. 2021; 6: 38-47.
- Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, et al. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn’s Disease. Gastroenterology. 2020; 158: 189-99.
- Ding NS, Hart A, De Cruz P. Systematic review: Predicting and optimising response to anti-TNF therapy in Crohn's disease - algorithm for practical management. Aliment Pharmacol Ther. 2016; 43: 30-51.
- Oh EH, Ko D-H, Seo H, Chang K, Kim G-U, et al. Clinical correlations of infliximab trough levels and antibodies to infliximab in South Korean patients with Crohn's disease. World J Gastroenterol. 2017; 23: 1489-96.
- Clinical Implications of Variations in Anti-infliximab Antibody Levels in Patients with Inflammatory Bowel Disease | Inflammatory Bowel Diseases | Oxford Academic.
- Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): A meta-analysis. Am J Gastroenterol. 2013; 108: 40-7; quiz 8.
- Anti-infliximab Antibodies with Neutralizing Capacity in Patients with Inflammatory Bowel Disease: Distinct Clinical Implications Revealed by a Novel Assay - PubMed.
- Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients with Inflammatory Bowel Diseases - ScienceDirect.
- Tournier Q, Paul S, Williet N, Berger A-E, Veyrard P, et al. Early detection of anti-drug antibodies during initiation of anti-tumour necrosis factor therapy predicts treatment discontinuation in inflammatory bowel disease. Aliment Pharmacol Ther. 2021; 53: 1190-200.
- The temporal evolution of antidrug antibodies in patients with inflammatory bowel disease treated with infliximab - PubMed.
- Tun GSZ, Downey R, Robinson K, Wright A, Marshall L, et al. High prevalence of antibodies to infliximab and their relation to clinical outcomes in inflammatory bowel disease patients. GastroHep. 2019; 1: 214-22.
- Strik A, Berends S, Mould D, Mathôt R, Ponsioen C, et al. DOP56 Dashboard driven vs. conventional dosing of infliximab in inflammatory bowel disease patients: the PRECISION trial. Journal of Crohn's and Colitis. 2019; 13: S063.
- Papamichael K, Cheifetz AS, Melmed GY, Irving PM, Vande Casteele N, et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clinical Gastroenterology and Hepatology. 2019; 17: 1655-68.e3.
- Maintenance infliximab for Crohn's disease: The ACCENT I randomised trial - PubMed.
- Long-term treatment of rectovaginal fistulas in Crohn’s disease: Response to infliximab in the ACCENT II Study - Clinical Gastroenterology and Hepatology.
- Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. 2014; 146: 392-400.e3.
- Cassinotti A, Travis S. Incidence and clinical significance of immunogenicity to infliximab in Crohn's disease: a critical systematic review. Inflamm Bowel Dis. 2009; 15: 1264-75.
- Gomes LEM, da Silva FAR, Pascoal LB, Ricci RL, Nogueira G, et al. Serum Levels of Infliximab and Anti-Infliximab Antibodies in Brazilian Patients with Crohn’s Disease. Clinics. 2019; 74.
- Farrell RJ, Alsahli M, Jeen YT, Falchuk KR, Peppercorn MA, et al. Intravenous hydrocortisone premedication reduces antibodies to infliximab in Crohn's disease: A randomized controlled trial. Gastroenterology. 2003; 124: 917-24.