Case Report - Volume 3 - Issue 5
Nocardia elegans mixed infection of pulmonary and cutaneous: A case report
Hongwei Zhang1; Yuedong Yue1; Bo Yu2; Guanzhou Feng3*; Xiaobin Fu1*
1Department of Pharmacy, Liao Cheng People’s Hospital, Liao Cheng, Shandong, China.
2Department of Respiratory, Liao Cheng People’s Hospital, Liao Cheng, Shandong, China.
3Department of Hospital office, Liao Cheng People’s Hospital, Liao Cheng, Shandong, China
Received Date : August 02, 2023
Accepted Date : Sep 13, 2023
Published Date: Sep 20, 2023
Copyright: © Xiaobin Fu 2023
*Corresponding Author: Xiaobin Fu, Department of Hospital office,
Liao Cheng People’s Hospital, Liao Cheng, Shandong, China.
Email: fgz020@126.com
DOI: Doi.org/10.55920/2771-019X/1552
Abstract
Background: Nocardia elegans mixed infection of pulmonary and cutaneous are exceedingly rare.
Nocardia asteroides, an aerobic, variably acid fast, Gram-positive bacteria, and disseminated nocardiosis is opportunistic infection mostly occurring in immunocompromised patients. This organism is almost always associated with pulmonary infection, and the number of case series in the literature is increasing.
Case Report: We present a case of disseminated nocardiosis caused by Nocardia elegans in a 44-year-old man with both pulmonary and cutaneous involvement, and discuss the therapeutic scheme of him. As with previously reported pulmonary and cutaneous infections, the current case was treated successfully with trimethoprim–sulfamethoxazole, carbapenems, and moxifloxacin. However, the clinical characteristics of this organism remain unclear.
Conclusion: Further studies are therefore required to develop more effective treatment protocols for disseminated nocardiosis, clinical pharmacists explored the characteristics and principles of treatment of Nocardia asteroides infection and we hope provide better pharmaceutical care and usefull antibacterial regimens for patients.
Keywords: Nocardia elegans, pulmonary infection, cutaneous involvement, trimethoprim–sulfamethoxazole, moxifloxacin
Background
Nocardia spp. is clinically important bacterial pathogens that cause suppurative infections ranging from localized lung or cutaneous involvement to disseminated disease. The number of sdhpmacases of pulmonary nocardiosis reported in the literature is increasing, and in some cases, a diagnosis is reached even postmortem [1,2]. The majority of the human Nocardia infections has been attributed to Nocardia asteroids and Nocardia brasiliensis, with Nocardia otitidiscavarium, Nocardia nova, Nocardia farcinica and Nocardia elegans known as uncommon pathogens [3,4,5]. In recent years, the number of case reports has been increasing and it is thought that the true incidence in immunocompetent and immunocompromised patients is increasing, it can disseminate to the kidneys, joints and other regions [6,7,8]. In China, most of the reported cases of nocardiosis are caused by N. asteroides and N. brasiliensis. Here we present a case of N. asteroides caused by N. elegans in a 44-year-old man from China.
Case Report
A 44-year-old man, weight 88kg, seeking medical attention for 5 months repeated fever with pain in the upper left arm, cough with phlegm for 1 month in our hospital in 2 Jan. 2018. He has presented with a three-year history of diabetes mellitus, nephrotic syndrome and bile duct stones, and treated with ciclosporin (50mg qd) and prednisolone (60mg qd) for two years duo to nephrotic syndrome, blood sugar was controlled by insulin aspart and glargine. In past 5 months, he has seen the doctors in different hospital for fever of unknown origin, follow-up chest CT scans showed that shadow and cavity in the right lung, but blood culture did not find the pathogenic bacteria, but fungal hyphae were identified in sputum.
The patient was diagnosed with 1. fever forunknown origin (FUO) (invasive pulmonary aspergillosis(IPA) ? or tuberculosis ?), 2. nephrotic syndrome, 3. diabetes mellitus, 4. bile duct stones in our hospital. Blood tests indicated neutrophil ratio (89.5 %), hematoglobin (26 g/L), procalcitonin (10ng/ml), hemameba (10.2×109/L), erythrocyte sedimentation rate (64mm/h), C-reactive protein (41.8mg/L), kidney malfunction (serum creatinine 123 umol/ml), procalcitonin (10ng/ml). Chest CT showed lung markings increased and multiple nodules in double lung, cavity in the upper lobe of the left lung, weak ground glass density in the inferior lobe of the double lung (Figure 1). The result indicated the pneumonia and interstitial pneumonia, and moxifloxacin (0.40g iv.drip qd), glargine
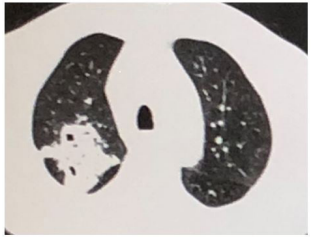
Figure 1: Large dense foci were seen in the posterior segment of the upper lobe of the right lung, multiple nodules and patchy high-density lesions were seen in both lungs.
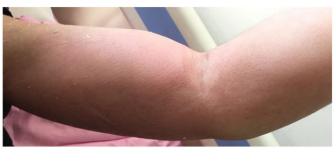
Figure 2: The left upper arm is red and swollen, skin temperature rises tall, ache.
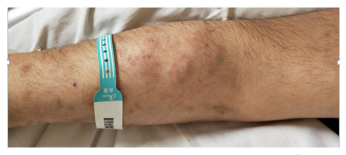
Figure 3: There were 5 abscesses with a diameter of 2cm in the right forearm.
(15u h. qn) + insulin aspart (12u-8u-10u h) preprandial injection and prednisone (25mg po qd) were administered for anti-infection, blood sugar controlling and nephritic syndrome respectively.
In 5 Jan., the patients with cough, sputum improved markedly, but the temperature rised again up to 38.4 ℃, a small amount of purulent secretion was seen in the upper lobe of the right lung under bronchoscopy, the mucosa was normal and the fluoroscopy was negative. The results of bacterial culture were streptococcus aeruginosa, negative for acid resistance staining, fungi and bacteria. In 6 Jan., the patient's temperature 38 ℃, left arm pain, the B ultrasonic examination suspected a cystic in the left upper limb forearm medial muscle in the size of about 17 cm x 2.9 cm, the shape was not regular. In 10 Jan., the patient’s temperature 38.5 ℃, the left arm swelling skin temperature was high, the pain was aggravating (Figure 2), and five small abscesses 2cm in diameter appeared in the right forearm (Figure 3). Blood biochemistry: creatinine (106.8umol/L). Patient's medical history indicated
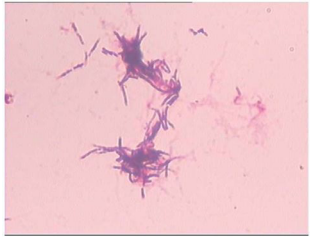
Figure 4: Nocardia was observed by HE staining.
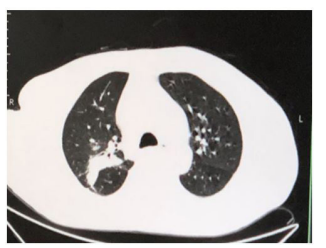
Figure 5: Increased texture in both lung, shadow in the posterior segment in the right upper lobe of lung.
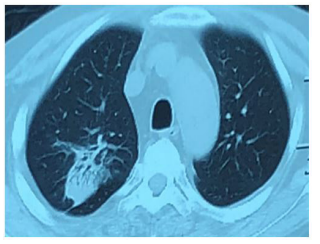
Figure 6: Spotted lamellae high density shadow in the basal segment in the right upper lobe and lower lobe.
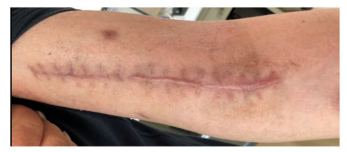
Figure 7: The incision heals well in the left upper arm.
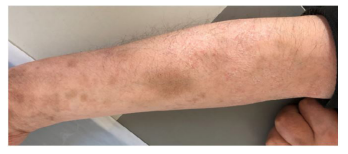
Figure 8: The abscess was completely absorbed in right forearm.
that left arm was red, there was no split on skin, and no history of trauma. The hand surgery department considered skin and soft tissue infection, and the patient was transferred to hand surgery department for further treatment. In the hand surgery department, the patient was treated with levofloxacin (0.5g ivdrip qd). In 11 Jan., arm abscess incision and drainage were performed, and the thick, yellow fluid obtained from the center of the lesion, exudates was applied to germiculture. Indwelling drainage tube, the amikacin (500 ml topical q8h 1 g + NS) was used to wash continually. In 12 Jan., the patient's temperature dropped to 38.0 ℃, postoperatively cefoperazone-sulbactam (3g ivdrip q12h) was treated to anti-infection. In 13 Jan., the patient's temperature 36.5 ℃, the bacterial culture result was star Nocardia (figure 4). In 15 Jan., the patient's temperature 37.6 ℃, the abscess on the right arm was not relieved, and the clinical pharmacists were consulted. The clinical pharmacists thought nocardia was pathogenic bacterium, and the sulfamethoxazole was the preference. The pharmacist recommended the temporary using of linezolid (600 mg ivdrip q12h), stopped using of cefoperazone
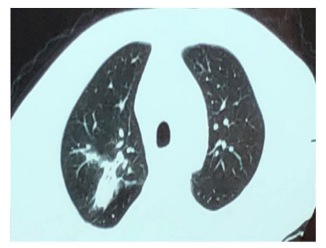
Figure 9: The irregular strip density decreased in the upper lobe of the right lung.
sulbactam for patients with nephrotic syndrome. The patient’s temperature down to normal and the abscess on the right arm was relieved at the second day of application of linezolid, however, the patient unable to eat normally with nausea and vomiting. Clinical pharmacists considered that gastrointestinal reaction was caused by linezolid,so that the doctor replaced the antibacterial drug with cefoperazone sodium sulbactam. In 20 Jan., the patient with fever again, the highest body temperature was 37.5 ℃. The clinical pharmacist suggested that compound sulfamethoxazole (0.96g, po, qid) should be used. Patients with significant anasarca, biochemical all items: total protein (50g/L), albumin (19g/L), creatinine (957umol/L). Urine routine: protein 3+, tube type count 6.05/ul, pathocast 3.5/LP, casts 17.55/LP. The consultation of the department of nephrology suggested that the dosage of hormone should be 30mg qd plus cyclosporine (100mg bid). In 24 Jan., the patient's temperature is normal, the chest CT (figure 5): double lung texture, solid shadow was seen in the upper lobe of the right lung, gas in bronchus, symptoms improved. In 27 Jan., body temperature was normal, right arm swelling decreased, and the patient was allowed to leave the hospital. Discharge diagnosis: 1. The Nocardia infection ((1) skin and soft-tissue infections (SSTIs) (2) lung infection?), 2. Nephrotic syndrome, 3. Diabetes, 4. Bile duct calculi. Discharge medication: taking compound sulfamethoxazole for at least 6 months (0.96 g, Po, qid), prednisone piece (30 mg qd).
Follow-up records
In 28 Jan., the patient’s body temperature is normal, right arm swelling decreased. Nausea and vomiting occurred after taking compound sulfamethoxazole (2 pieces, tid) the medicine three times, patient changed the dosage of compound sulfamethoxazole to (1 pieces, tid). In 5th Feb., temperature is normal, no nausea vomiting or diarrhea, sutures were removed on the left arm, incision was healing well. The mass in the right forearm became smaller and flatter. In 10 Feb., chest CT (figure 6): patchy dense shadows were seen in the upper lobe and lower basal segment of the right lung. In 12th Feb., the lumps in right forearm disappeared. In 30 Mar., the patient went to the hospital to review: the patients with good spirit, incision healing well in left arm (figure 7). The abscess in right forearm was absorbed completely (figure 8). Chest CT again showed irregular mass density increased in the upper lobe of the right lung, the area was smaller than that of 24, 01, 2018 (figure 9). Enlarged lymph nodes were seen in the mediastinum and hilus pulmonis with local dilatation of intrahepatic bile duct.
Discussion
Nocardia species are ubiquitous environmental microorganisms that are found worldwide and belong to a diverse group of bacteria known as aerobic actinomycetes. The most common of these include Nocardia asteroides sensu stricto, Nocardia brasiliensis, Nocardia farcinica, Nocardia nova, Nocardia otitidiscaviarum, and Nocardia transvalensis [6,7,8]. In most instances, Nocardia is an opportunistic pathogen, with the majority of infections occurring in immunocompromised hosts [9,10]. At present, Gram staining is the only method for the rapid diagnosis of Nocardia infection. Culture of this pathogen requires several days to several weeks, and continuation of culture is difficult due to overgrowth of other bacteria unless their growth is kept in check. It was reconfirmed that Gram staining is the most important method for achieving not only an early diagnosis and treatment but also improving the success rate of culture and administering optimum treatments based on drug susceptibility. There is usually a delay in its diagnosis, therefore, in specific treatment, worsening the prognosis for an illness that already has a high rate of mortality. In addition, the presence of chronic respiratory disease, such as chronic obstructive pulmonary disease, is a risk factor of pulmonary nocardiosis [11-15].
In our case, the patient had recurrent fever for two months, the patient had been kept for blood collection and sputum culture for many times in hospital, however, no pathogenic bacteria were detected. Meanwhile, the pathogenic bacteria in tracheoscopy lavage and skin secretions of the hospitalized patient were also not detected. The patients with nephrotic syndrome, taking prednisone and ciclosporin for 3 years, and also with diabetes, who has high-risk factors of the causing of nocardia infection. The patients hospitalized from lung infection and high fever, the skin and soft tissue infection of the left arm was aggravated during the treatment. With left arm abscess incision operation, the thick yellowish-brown pus was seen intraoperatively, and bacterial culture revealed nocardia. We highly suspect that the patient has been infected with astrocyte nocardia for 5 months.
The primary drug for nocardia caused lung infection is compound sulfamethoxazole (trimethoprim sulfamethoxazole, TMP-SMZ) joint imipenem, the secondary drug is linezolid, amikacin joint imipenem is also effective [16,17]. The dose of compound sulfamethoxazole of the patient was calculated as 3mg/ (kg. d) according to TMP, which was relatively low, mainly because patient suppressed appetite significantly after application of high-dose [18,19]. Small-dose treatment was effective and no other adverse drug reactions occurred except for skin rash, and there was no relapse [20].
Multi-site infection caused by Nocardia is rare and easy to be misdiagnosed or missed. In our case, the patient was diagnosed after repeated fever for 5 months, so that the patients with low immunity must be vigilant. Due to the slow growth of Nocardia, the attention should be paid to the pathogenic examination of suspected cases. Also, the clinical pharmacists comprehensively studied the biological characteristics, pathogenicity and treatment of nocardia through drug monitoring of patients, meanwhile, analyzed and discussed the variety, dose and course of antibacterial drugs. Clinical pharmacists removed recurrent fever that has been troubling patients for a long time, and provided better pharmaceutical services, which improve the satisfaction of patients and the professional level of clinical pharmacists.
Funding
This work was supported by Clinical Pharmaceutical Research Special Fund of Shandong Medical Association (YXH2021ZX022).
References
- Luo N, Tan S, Li X, Liu S, Singh S, Chen M, et al. Pulmonary nocardiosis in a patient with pemphigus foliaceus: case report and literature review. BMC Infect Dis. 2021; 21(1): 8.
- Taylor S, Brown TL, Tucci J, Lock P, Seviour RJ, Petrovski S. Isolation and characterization of bacteriophage NTR1 infectious for Nocardia transvalensis and other Nocardia species. Virus Genes. 2019; 55(2): 257-65.
- You Y, Chen W, Zhong B, Song Z, Yang X. Disseminated nocardiosis caused by Nocardia elegans: a case report and review of the literature. Infection. 2018; 46(5): 705-10.
- Nakamura I, Nagakura T, Fujita H, Fukusima S, Gonoi T. Nocardia elegans infection: a case report and literature review. Int J Infect Dis. 2017; 54: 15-17.
- Matsubayashi S, Iikura M, Numata T, Izumi S, Sugiyama H. A case of Aspergillus and Nocardia infections after bronchial thermoplasty. Respirol Case Rep. 2018; 7(2): e00392.
- Duggal SD, Chugh TD. Nocardiosis: A Neglected Disease.Med Princ Pract. 2020; 29(6): 514-23.
- González-Jiménez P, Méndez R, Latorre A. Pulmonary Nocardiosis. A case report. Rev Esp Quimioter. 2022; 35 Suppl 1(Suppl 1): 114-116.
- Khorshidi M, Navid S, Azadi D, Shokri D, Shojaei H. A case report of brain abscess caused by Nocardia cyriacigeorgica in a diabetic patient. JMM Case Rep. 2018; 5(9): e005133.
- Brown-Elliott BA, Wallace RJ Jr. In Vitro Susceptibility Testing of Tedizolid against Isolates of Nocardia. Antimicrob Agents Chemother. 2017; 61(12). pii: e01537-17.
- Hemmersbach-Miller M, Catania J, Saullo JL. Updates on Nocardia Skin and Soft Tissue Infections in Solid Organ Transplantation. Lerner PI. Curr Infect Dis Rep. 2019; 21(8): 27.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996; 22(6): 891-903
- De Farias MR1, Werner J, Ribeiro MG, Rodigheri SM, Cavalcante CZ, Chi KD, et al. Uncommon mandibular osteomyelitis in a cat caused by Nocardia africana. BMC Vet Res. 2012; 8: 239.
- Soares D, Reis-Melo A, Ferraz C, Guedes Vaz L. Nocardia lung abscess in an immunocompetent adolescent. BMJ Case Rep. 2019; 12(1). pii: bcr-2018-227499.
- Ninan MM, Venkatesan M, Balaji V, Rupali P, Michael JS. Pulmonary nocardiosis: Risk factors and species distribution from a high burden centre. Indian J Med Microbiol. 2022; 40(4): 582-84.
- Takiguchi Y, Ishizaki S, Kobayashi T, Sato S, Hashimoto Y, Suruga Y, et al. Pulmonary Nocardiosis: A Clinical Analysis of 30 Cases. Intern Med. 2017; 56(12): 1485-90.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for nocardia infections. Expert Opin Pharmacother. 2013; 14(17): 2387-98.
- Kurahara Y, Tachibana K, Tsuyuguchi K, Akira M, Suzuki K, Hayashi S. Pulmonary nocardiosis: a clinical analysis of 59 cases. Respir Investig. 2014; 52(3): 160-6.
- Fujita T, Ikari J, Watanabe A, Tatsumi K. Clinical characteristics of pulmonary nocardiosis in immunocompetent patients. J Infect Chemother. 2016; 22(11): 738-43.
- Uhde KB1, Pathak S, McCullum I Jr, Jannat-Khah DP, Shadomy SV, Dykewicz CA, et al. Antimicrobial-resistant nocardia isolates, United States, 1995-2004. Clin Infect Dis. 2010; 51(12): 1445-8.
- Mehrabadi SM, Taraghian M, Pirouzi A, Khaledi A, Neshani A, Rashki S. Pulmonary Nocardiosis in Suspected Tuberculosis Patients: A Systematic Review and Meta-Analysis of Cross-Sectional Studies. Ethiop J Health Sci. 2020; 30(2): 293-300.

