Case Report - Volume 3 - Issue 5
Particularities in cutaneous filariasis
Cristina Stanescu*; Georgiana-Daniela Stanescu; Ioana Tamas; Camelia Tamas
1Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, Dunarea de Jos University, 35 Alexandru Ioan Cuza Street, 800216 Galati, Romania.
2Department of Plastic Surgery, Faculty of Medicine, Grigore T. Popa University of Medicine and Pharmacy, 16 Universitatii Street,700115 Iasi, Romania.
3Department of General Surgery, Faculty of Medicine, Grigore T. Popa University of Medicine and Pharmacy, 16 Universitatii Street,700115 Iasi, Romania.
Received Date : Sep 06, 2023
Accepted Date : Sep 30, 2023
Published Date: Oct 11, 2023
Copyright: © Cristina Stanescu 2023
*Corresponding Author : Cristina Stanescu, Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, Dunarea de Jos University, 35 Alexandru Ioan Cuza Street, 800216 Galati,Romania.
Email: cristina.stanescu@ugal.ro
DOI: Doi.org/10.55920/2771-019X/1566
Abstract
Filariasis are a type of parasites (worms), who have a cylindrical body and sexual dimorphism. They are part of the filarioid family. The epidemic zone for filariasis is located in Central, as well as the West part of Africa. There are many species of filariasis, but the cutaneous filariasis (Loa-Loa, Onchocerca volvulus, Dracunculus medinensis) and lymphatic filariasis (Wucherelia bancrofti, Brugia malayi) are of medical interest. Other species of filariasis can cause incomplete infections due to the incapacity of reaching a certain complet level of maturity in the human body. Human transmission is made with the help of the red fly (Chrysops-Tabanid), which can take the role of an intermediary host and carrier [1]. Some microfilariae have a daily circadian rhythm, periodically in which they are found in maximum concentration in the bloodstream. These intervals match with the feeding circadian rhythm of the insects with the role of a carrier, maximizing this way, the chances of spreading the infection [2]. We are presenting the case of a pacient, diagnosed postoperatively with cutaneous filariasis, with insidious onset after an insect bite and the installation of local inflamatories events, which have progressively worsened, despite treatments at home, which is why sugery was performed (surgical debridement and removal the parasite).
Keywords: Cutaneous filariasis; Risk factors; Diagnosis; Treatment; Prognosis.
Introduction
The larva in the third stage of development is inoculated into a vertebrate organism during the insect’s feeding through a sting, after which it crosses the dermis and enters to the lymphatic vessels. In humans the life cycle of the parasite lasts between five to 12 months and in the vector between 10 and 12 days. Over a period of 9 month the larva transform into an adult worm with dimensions between 20 and 100 milimeters. In general the parasite can survive for about 5 years [1]. When it reaches maturity, the parasite eliminates the microfilariae that migrate in the peripheral blood, cerebrospinal fluid and sputum during the day, and in the lungs during the night. Adult worms tend to move subcutaneously and have tactics for the eyeball, locating subconjunctivally. The tendency to migrate from one eye to another, causing conjunctivitis, can also be observed. The disease minimal incubation lasts between 4-5 months, but it can extend to 1-2 years [2].
The most common symptom is localized or extensive allergic edema in the upper and lower limbs. Edema of cutaneous filariasis is fleeting, lasting up to 36-72 de ore, slightly painful, accompanied by hyperesthesia and local itching, which can generalize [3]. Other complications that may occur in cutaneous filariasis are abscesses of subcutaneous tissue, tenosynovitis, eosinophilic fasciitis, lymhadenophaty, elephantiasis, ocular complications (retinophaty, conjunctivitis), neurological (dizziness, headache, coma), cardiovascular (cardiomyopathy), renal (glomerulonephritis, chronic, proteinuria) or pulmonary complications. Frequently patients may be asymptomatic and have only eosinophilia [4].
Filariasis may be suspected in patients who have travelled to endemic areas or who have specific symptoms (allergic edema, febrile syndromes, adult worms at subconjunctival or subcutaneous level). Laboratory tests showing the presence of eosinophilia and elevated C-reactive protein values are required to establish a definite diagnostic. Hematological diagnosis identifies the presence of microfilariae in the peripheral blood using Giemsa or methylene blue coloration. Immunological diagnosis identifies elevated levels of specific IgE and IgG4 antibodies by Elisa technique [5]. The histopathological diagnosis consists in recognition by the anatomopathologist of the adult worm after its extraction from subconjunctivalk or subcutaneous level. If internal organs are affected, other imaging investigations can be performed to detect the associated lesions (computer tomography, nuclear magnetic resonance, soft tissue ultrasound etc).
The surgical treatment consist of extracting the adult worm from the eye ball or from the subcutaneous tissue, surgery performed under local anesthesia. Rupture of the filaria or its incomplete extraction is associated with important local and general hypersensitivity reactions, but also an increased risk of infection. Postoperatively patients will be treated with Diethylcarbamazine, Ivermectin or Albendazole in doses prescribed by a specialist [6].
Treatment with antiparasitic drugs is administered in progressively increasing doses. These drugs have the role of eliminating the larval worm by inhibiting reproduction and destroying it. Side effects caused by these drugs can be tempereted by concomitant administration of antihistamines or steroidal antiinflamatory drugs. The elimination of worms must be done with caution because a very high concentration of them in the blood or lymphatic flow can cause violent allergic reactions and lymphadenopathy [7].
Diethylcarbamazine is an affective treatment against filariasis with effect on adult worms and is prescribed only after confirmation of the diagnosis. Treatment has a significant risk of allergic reactions, much higher than Ivermectin, but with a lower risk of neurological comlications. In patients with specific symptoms of cutaneous filariasis and with less than 8000 microfilariae/ml of blood, is recommended 3-5 mg of Diethylcarbamazine/kg body weith/day for a period of 21 days. Ivermectin is also used to reduce the number of microfilariae, but under the protection of antihistamines, repeated monthly for 4 months. Because side effects of ivermectin can be severe, especially in neurological field (headache, ataxia, confusion, sphincter incontinence, encephalopathy, central hypertension, coma); lately Albendazole is preferred because it has lower risks [6].
The prognosis in filariasis depends on how large the number of pathogens is and the period of contact. During the disease the immune system is affected and the body has a predisposition to other diseases. Especially in tropical regions, subsequent infections can lead to severe life-threatening complications. Adults worms can survive in the host s body for many years. It may take several months or years for the microfilariae to appear in the blood, so that the infection can be diagnosed late [8]. An early treatment is associated with a better prognosis. In lymphatic filariasis lymphatic vessels can be destroyed, lymphatic drainage being affected, with significant aesthetic and functional damage.
Case Presentation
A 74 year old patient from urban environment presented in plastic surgery with a nodular subcutaneous formation, located at the level of righ nasogenic region, of firm consistency, pruritic, slightly sensitive to palpation, adherent to superficial planes, mobile on deep plans (Figure no 1). The onset was insidious, after an insect sting about 4 months ago, with the persistence of a local erythema and later with the onset of pruritus, phenomena that have progressively worsened with the deformation of the region.
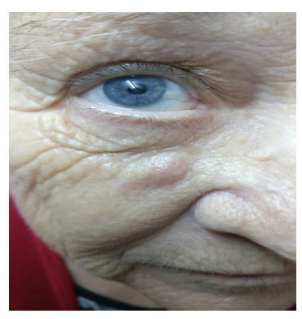
Figure 1: Preoperative aspect.
The patient had a febrile episode in the last 2 months, which resolved after the administration of antibiotics and anti-inflammatory of the family doctor indication. Due to the progressive increase in the size of the subcutaneous nodule and the local pruritus, the patient presented to the outpatient clinic for plastic surgery. Preoperative tests showed mild leukocytosis with eosinophilia. The diagnosis of supposition was of infected epidermal cyst and surgery was scheduled, which was performed under local anesthesia, practicing the incision of the skin, evacuation of secretions and extraction of the parasite, in the form of a clot (figure 2). Histopathological examination confirmed the diagnosis of cutaneous filariasis.
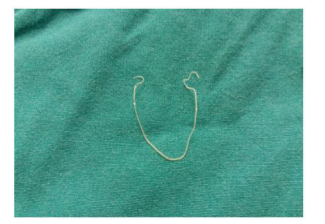
Figure 2: Parasite extracted.
Postoperatively, the evolution was favorable (figure 3) and the patient was refered to the infectious disease ward for monitoring and specialized treatment.
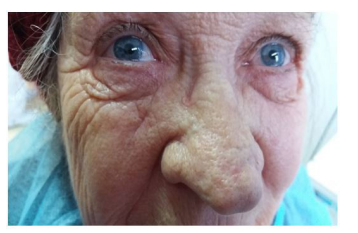
Figure 3: Postoperative aspect.
The patient reported that she did not have contact with people who had returned from abroad.
Conclusion
Most often the diagnosis of cutaneous filariasis is made postoperatively after the parasite has been removed. Serological examination may be suggestive of diagnosis when we have a suspicion of cutaneous filariasis. The prognosis is favorable when the diagnosis is early and the removal of the parasite is performed before the elimination of circulating microfilariae.
References
- Dreyer G, Noroes J, Figueredo-Silva J. New insights into the natural history and pathology of bancroftian filariasis: implications for clinical management and filariasis control programmes. Trans R Soc Trop Med Hyg. 2000; 94(6): 594-6.
- Dreyer G, Noroes J, Figueredo-Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: a clinical perspective. Parasitol Today. 2000; 16(12): 544-8.
- Fox LM, Furness BW, Haser JK, Brissau JM, Louis-Charles J, Wilson SF, Addiss DG, Lammie PJ, Beach MJ. Ultrasonographic examination of Haitian children with lymphatic filariasis: a longitudinal assessment in the context of antifilarial drug treatment. Am J Trop Med Hyg. 2005; 72(5): 642-8.
- Gorbach SL, Bartlett JG, Blacklow NR. Eds. Infectious Diseases. W.B. Saunders Company, Philadelphia, PA. 1992.
- Mandell GL, Bennett JE, Dolan R. Eds. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 4th ed. Churchill Livingstone Inc. New York, NY. 1995.
- Del Giudice P, Chosidow O, Caumes E. Ivermectin in dermatology. J Drugs Dermatol. 2003; 2: 13-21.
- Horton J. Albendazole: a broad spectrum anthelminthic for treatment of individuals and populations. Curr Opin Infect Dis. 2002; 15: 599-608.
- Shenoy RK. Management of disability in lymphatic filariasis-an update. J Commun Dis. 2002; 34: 1-14.
- Lammie PJ, Cuenco KT, Punkosdy GA. The pathogenesis of filarial lymphedema: is it the worm or is it the host? Ann N Y Acad Sci. 2002; 979: 131-42; 188-96.
- Weil GJ, Lammie PJ, Weiss NW. The ICT Filariasis Test: A Rapid-format Antigen Test for the Diagnosis of Bancroftian Filariasis. Parasitol Today. 1997; 13: 401-05.

