Case Report - Volume 3 - Issue 5
Anaplasic large cell lymphoma ALK+: Anatomo-pathological characterization of three pediatric cases
Gabriel Cao 1,2*; Julian Mendez 1; Graciela Ottaviano2; Francisco Capani2
1Anatomical Pathology Division, Pedro de Elizalde Children’s Hospital, Avenida Montes de Oca 40 (C1270AAN), Buenos Aires, Argentina.
2CAECIHS - Centro de Altos Estudios en Ciencias Humanas y de la Salud, UAI. National Council of Scientific and Technical Research
(CONICET), Av. Montes de Oca 745, 2º piso - (C.P. 1270AAH), Buenos Aires, Argentina.
Received Date : Sep 21, 2023
Accepted Date : Oct 06, 2023
Published Date: Oct 13, 2023
Copyright: © Gabriel Cao 2023
*Corresponding Author : Gabriel Cao, HGN “Pedro de Elizalde”, Montes
de Oca Avenue 40, C1270AAN Autonomous City of Buenos Aires (Argentina).
Email: drgabrielcao@hotmail.com
DOI: Doi.org/10.55920/2771-019X/1569
Abstract
The anaplastic large cell lymphoma ALK+ (ALCL ALK+) is a rare malignancy in childhood, which normally occurs in advanced clinical stages. The common histological subtype is composed by large cells with cytomorphology and immunohistochemistry feature of CD30 and T markers expression. The neoplasia presents a translocation t (2; 3) (p23; q35) related to up- regulated of kinase associated with anaplastic cells (ALK), whose overexpression is detectable by immunohistochemical techniques (ALK-1). The presence of lymphadenopathies and prolonged fever can simulate an infectious process. The presence of B symptoms, extranodal, mediastinal, visceral and cutaneous involvement, significantly reduce the prognosis of the patient. Medullary infiltration at the time of the diagnosis, together with the histological subtypes of small cells and lymphohistiocytic neoplasm, favouring frequent relapses. Treatment is based on different regimens and combine cytarabine, methotrexate, doxorubicin, prednisolone, vincristine, and 6-mercaptopurine. Other therapies include the use of an anti-CD30 antibody and an ALK enzyme inhibitor drug.
keywords: Anaplastic large cell lymphoma; ALK +; paediatrics
Introduction
The anaplastic large cell lymphoma (ALCL) is a set of malignancies inside of the non-Hodgkin lymphomas, mostly with histopathologic and immunohistochemical features like cells of T origin. One of their first descriptions was made in 1985 by Stein et al, who presented a series of cases consisting of lymphomas composed of large and pleomorphic cells capable to express Ki-1 (CD30), considering it an entity derived from activated lymphocytes, probably cytotoxic T cells [1]. Interesting, a group of ALCL´s have a chromosomal translocation involving the kinase of the anaplastic lymphoma (ALK), whose protein derivative can be detected by immunohistochemistry. The WHO 2008 ranking, reviewed in 2016, discriminates three biological entities: ALCL (ALK +), ALCL (ALK) and primary ALCL in skin [2, 3]. The first two are systemic neoplasms, with nodal or extranodal involvement, while the last is typical of the skin and lacks ALK expression. Although 90% of LACGs are considered mature T cell-derived neoplasms, they sometimes lack the clonal rearrangements corresponding to T surface receptors. Also, has not been shown definitively the possible cell of origin in cases with "null" phenotype [4]. The ALCL (ALK +) is rare, representing the 3% of non-Hodgkin lymphomas in adults and 10-20% of paediatric cases, with a predominance male to female ratio of 1.5 -2: 1 [ 5- 7].
The aim of this study is to characterize ALCL (ALK +) in three paediatric patients, one with nodal and extranodal compromise, while the remaining two cases were diagnosed by biopsy of axillary and cervical lymphadenopathy, respectively.
Clinical Cases
Case 1: Patient of 7-year-old, male, derived from another institution, presented prolonged fever, bilateral pleural effusion, cervical lymphadenopathy, fatigue, weakness, weight loss and anti - tuberculosis treatment for three months, without clinical improvement. It progressed with abdominal pain and absence of catharsis, whereby and it did abdominal ultrasound showing diffuse thickening of the wall of the colon descending and sigmoid, as well as presence of ascitic liquid in the left parietocolic space, Morrison space and right lower quadrant. CT with and without contrast showed bilateral cervical lymphadenopathies, in the anterior mediastinum and retroperitoneum. The clinical and serological laboratory tests did not show pathological alterations (Fig. 1A – 1C). During hospitalization, tuberculosis was ruled out, what's more an exploratory laparotomy was performed by intestinal obstruction, and the induration of the greater omentum was verified, obtaining, by partial omentectomy, three irregular white-yellowish fragments, which together measured 8 x 3 cm and weighed 15 grams. In the largest of them, at one end, a hard-elastic nodular sector was identified, measuring 3.5 x 2.5 x 2 cm. The cut surface was yellowish with whitish sectors of geographical contours.
Pathology showed fibroadipose tissue infiltrated by malignant large anaplastic cells, with moderate to marked anisocariosis, prominent nucleoli and presence of mitotic images typical and atypical in many sectors. Wide basophilic and occasionally vacuolated cytoplasms was frecuent (Fig. 2 C). Immunostaining techniques were performed, resulting positive for CD45 (ACL), CD3, CD5, CD30, ALK-1 and Ki67 (80% of the cell population) and negative for CD1a, CD20, CD68, CD45Ro, EMA, CK (AE1-AE3), Vimentin, Desmin and Neuronal Specific Enolase (NSE). Subsequently, a bone marrow biopsy was sent without pathological involvement. These findings support the diagnosis of ALCL (ALK +). The patient received chemotherapy with Vincristine, Actinomycin D, Ifosfamide , Doxorubicin, and MESNA, progressing unfavourably until death.
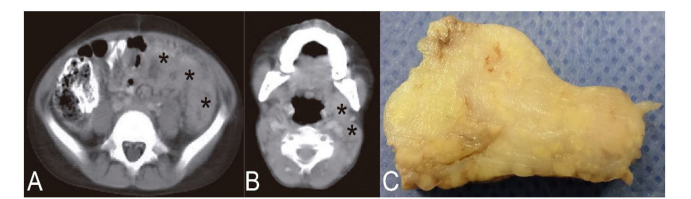
Figure 1: A, abdominal CT corresponding to Case 1 showing colonic parietal thickening (asterisks); B, cervical image of the same patient showing laterocervical lymphadenopathy (asterisks); C, After the exploratory laparotomy performed, three greater omentum fragments were obtained, one of which stands out in the image, with a yellowish-white color and a firm appearance.
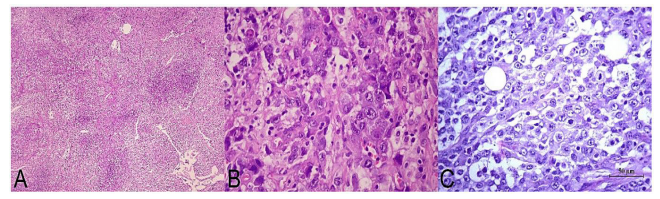
Figure 2: A, histological image at medium magnification corresponding to a biopsied adenopathy (Case 3) where distortion of the histoarchitecture is observed due to the presence of a malignant neoplastic proliferation of large cells (H&E, 100X); B, at higher magnification, details of the cell population can be seen, made up of large elements, nuclei with scattered chromatin occasionally notched, visible nucleolus, irregular nuclear membranes and ample cytoplasm, mostly eosinophils and eventually clear (H&E, 400X); C, histological image corresponding to the partial omentectomy in Case 1, showing diffuse tissue infiltration by large neoplastic cells that focally demonstrate numerous mitoses (H&E, 400X).
Case 2: Patient of 14 years old, male, with history of fever, functional impotence in left upper limb, fatigue, weakness, and weight loss of 17 kg in three months. Received different antibiotics schemes without favourable clinical response. The boy evolved with left axillary and supraclavicular lymphadenopathies, treated again with antibiotics to which he did not show therapeutic response. Subsequently, a lymph node biopsy was performed, which was reported as a “nonspecific lymphoproliferative process”. In such circumstances, the patient was referred to our Institution. He was afebrile, with laboratory parameters without particularities but positive IgG serologies for toxoplasma, parvovirus, cytomegalovirus, Epstein-Barr virus, and chickenpox. Blood and urine cultures performed were negative. Cervical ultrasound showed multiple bilateral lymphadenopathies predominantly on the left, extending into the supraclavicular and axillary spaces, increasing the echogenicity of the adjacent soft tissues. The chest CT with contrast also confirmed the presence of mediastinal lymphadenopathies and bilateral pleural effusion. With both methods, no abdominal pathology was observed. Finally, a biopsy of the left supraclavicular adenopathy was performed, obtaining a whitish wedge-shaped fragment measuring 1.5 x 1 x 0.5 cm. Histopathology demonstrated a malignant neoplastic proliferation, consisting of large cells, extensive cytoplasms, moderate to marked anisokaryosis, prominent nucleoli, and numerous atypical mitotic figures. With immunostaining techniques, neoplastic cells were positive for CD45 (ACL), CD30, CD3, ALK1 and focally EMA, while demonstrated negativity for CD45Ro, CD20, PAX5, CK (AE1-AE3), vimentin, desmin and NSE. The diagnosis of ALCL (ALK +) was made. The patient continued his treatment and follow-up at the original institution, but the subsequent evolution being unknown.
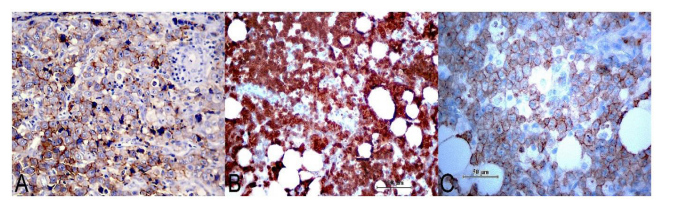
Figure 3: A, immunohistochemical expression for CD30 in neoplastic cells, with membrane distribution and occasionally paranuclear (IHC, 400X); B, Neoplastic proliferation strongly expressed ALK - 1 both in the cytoplasm and in the nucleus, a pattern determined by the ALK-NPM translocation (IHC, 200X); C, the neoplasm frequently expressed T markers, as was the case with CD5 observed in the image (IHC, 400X).
Case 3: A 13- year-old male patient who began with fever and non-painful bilateral cervical and supraclavicular lymph nodes, without response to antibiotic treatment, who was submitted to our Institution for study and diagnosis. The laboratory and serologic parameters were normal. A cervical ultrasound was performed, verifying bilateral polyadenomegaly. The CT scan with and without contrast, also showed bilateral mediastinal, axillary, and inguinal lymphadenopathies, in addition to retroperitoneal and bilateral pleural effusion. A 3 x 1 cm axillary adenomegaly was surgically biopsied, showing a malignant neoplastic proliferation of large cells, with hyperchromatic nuclei and prominent nucleoli (Fig. 2A and B). With immunohistochemical technique they were positive for CD45 (ACL), CD30, CD3, CD45Ro, ALK-1 and negative for vimentin, CD20, CK (AE1-AE3) and S100 protein. The entity was diagnosed as ALCL (ALK +). The patient received a treatment scheme consisting of vinblastine, ifosfamide, MESNA, etoposide, cytarabine, methotrexate and leucovorin, with poor evolution, dying two months after diagnosis.
Discussion
In paediatrics, over 95% of ALCL are (ALK +), with a mean age of presentation of 12-year-old [8]. In this age group, 75% of cases present in advanced stages of the disease, particularly in stages III-IV according to the St Jude classification for paediatric non-Hodgkin lymphomas. It is frequently associated with B symptoms, elevated febrile records [9], polyadenopathy, mediastinal involvement, and extranodal infiltration in soft tissues, lung, liver, skin, and bone. In biopsies of bone marrow stained with routine techniques, minor than 15% of cases evidence extranodal infiltration upon diagnosis, although this value can be increased to 30% with the use of immunohistochemistry techniques and 50% employed RT - PCR technique for the detection of the derivative protein of the ALK chromosomal translocation, both, in peripheral blood and bone marrow [10, 11]. On the other hand, involvement of the central nervous system and digestive tract are uncommon. The entity may coexist or be preceded by a hemophagocytic syndrome [12]. In rare cases, regional lymph node and skin involvement by ALCL (ALK +) has been observed after insect bites [13].
In the presented cases, they were male children, aged between 7 and 14 years, who demonstrated common clinical characteristics such as fever, polyadenopathy, mediastinal involvement and, in case 1, extranodal extension in the abdominal cavity. Likewise, all cases previously received different antibiotic regimens, without satisfactory clinical response.
Although LACG (ALK +) has a broad morphological spectrum, in all cases there is a variable proportion of large cells. This cell type is considered characteristic of the entity, given that appears in all histological variants. In this sense, different patterns were described: common (60% of cases), lymphohistiocytic (10%), small cells type (5-10%), Hodgkin-like (3%) that can simulate nodular sclerosis of classical Hodgkin lymphoma and, finally, a pattern of compound type (15%) [14, 15]. In the most frequently observed histological type, large neoplastic cells are present, with extensive cytoplasms that can be clear, basophilic, or eosinophilic. Furthermore, they are frequently multinucleated, with dispersed chromatin and evident nucleoli, sometimes simulating Reed Sternberg cells. In these cases, partial involvement of a lymph node by the LACG (ALK +) requires a differential diagnosis with a metastasis of carcinoma [16]. The lymphohistiocytic pattern is characterized by the presence of many reactive macrophages that accompany the neoplastic cells, being able to simulate an inflammatory process [17]. Furthermore, in the pattern of small cells predominates a neoplastic cell population of small to medium size, with central and irregular nucleus, obvious nucleolus, clear cytoplasm and often grouped surrounding the blood vessels. The conventional morphologic evaluation can confuse them with peripheral T lymphoma [18]. Although the previous histological variants are well defined, cases with more than one morphological pattern have been described [14] or with histological characters different from the previous ones [19, 20].
The differential diagnosis between the common variant of LACG (ALK +) and the histological subtypes of Hodgkin's lymphoma (HL) is important, especially in samples from cervical lymph nodes. In conventional microscopy, some features help guide the diagnosis. For example, in LACG (ALK +), neoplastic cells present greater cytological atypia than HL, associated with a extensive necrotic phenomena. The accompanying cellularity demonstrated in HL is not usually observed in LACG (ALK +). Immunohistochemistry contributes significantly to the diagnosis. In HL, neoplastic cells co-express CD30 and CD15, contrary to LACG (ALK +). Likewise, the expression of CD30 in LACG (ALK +) is more intense than HL. Of course, ALK1 is negative in Hodgkin cells, Reed Sternberg cells and Reed Sternberg like-cells. Sternberg cells, and Reed Sternberg like cells.
The immunohistochemical technique demonstrates that large neoplastic cells strongly express CD30 both, in the cytoplasmic membrane and in the Golgi zone [21], while in the small cell variant, the expression may be low or absent. In cases with a lymphohistiocytic pattern, CD30 expression is only found in larger neoplastic cells, which tend to be organized in small perivascular groups [16]. The development of an anti-ALK monoclonal antibody allowed the identification of LACG (ALK +) without the need for additional molecular studies. In this sense, the most frequently found genetic alteration is the t (2; 3) (p23; q35) translocation between the ALK gene located on chromosome 2 and the nucleophosmin gene (NPM) located on chromosome 5. Variations of the translocation fenomena can occur, involving to the chromosomes [1, 2, 3, 17, 19, 22] and X causing, in all cases, up- regulation of ALK [22]. In such circumstances, immunohistochemical expression will be cytoplasmic and nuclear, however, in the small cell variant, the marking is predominantly nuclear [5]. Frequently, neoplastic cells express T lineage antigens, although they may be loss of this characteristic, acquiring a “null” phenotype [23] (Fig. 3A – 3C). Also, this neoplasia can show variably immunolabel for CD45, CD45Ro and occasionally epithelial membrane antigen (EMA) and cytotoxic cell self-antigens. However, the expression for CD25 (IL-2 receptor) is usually intense [24, 25].
In the presented cases, the histological pattern corresponded to the common type, with immunohistochemical expression for CD45, CD30 and ALK-1. Likewise, all demonstrated immunostaining for T markers (CD3, CD5 and CD45Ro). The case 2 is unique, because focal expression for EMA was showed.
Important negative prognostic factors were associated to LACG (ALK +), such as the mediastinal, visceral (liver, spleen, lung), cutaneous involvements and B symptoms [26, 27]. Both, the progression-free survival, and the overall survival were reduced in cases with at least one clínical factor of risk [27]. Curiously, 12% of children and adolescents can develop a hemophagocytic syndrome, without negatively influencing the evolution [28]. Small cell and lymphohistiocytic variants as well as medullary infiltration at diagnosis are risk factors for disease relapse [26]. Chemotherapy regimens combine cytarabine, methotrexate, doxorubicin, prednisolone, vincristine, and 6-mercaptopurine. Other therapies include brentuximab vedotin, an antibody directed against the CD30 transmembrane glycoprotein receptor , and crizotinib , an ALK inhibitor [29].
Conclusion
The ALCL (ALK +) is a haematological malignancy uncommon in children, usually seen in advanced clinical stages. Symptoms B, mediastinal, visceral and cutaneous involvement are presented as poor prognostic factors. Small cell histologic subtypes, lymphohistiocytic, and medullary infiltration increase the risk of disease relapse. As first to line treatment different schemes that combine different drugs are used chemotherapeutic. Although the knowledge of this entity has expanded in recent decades, a greater number of studies in paediatric populations are necessary to standardize the therapy.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Authors' contributions
All authors participated in the bibliography search and in the preparation of the manuscript. All the authors approved the final version of it.
References
- Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985; 66 (4): 848-858.
- Eyre TA, Khan D, Hall GW, Collins GP. Anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: current and future perspectives in adult and paediatric disease. Eur J Haematol. 2014; 93(6): 455- 468.
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127(20): 2375- 2390.
- Xing X1, Feldman AL. Anaplastic large cell lymphomas: ALK positive, ALK negative, and primary cutaneous. Adv Anat Pathol. 2015; 22(1): 29-49
- Stein H, Foss HD, Dürkop H, Marafioti T, Delsol G, Pulford K et al. CD30 (+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features . Blood. 96(12), 3681- 36 95
- Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. (2004) Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2000; 15(10): 1467-1475.
- Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26 (25): 4124- 4130.
- Lowe EJ1, Gross TG. Anaplastic large cell lymphoma in children and adolescents. Pediatr Hematol Oncol. 2013; 30(6): 509-519
- Falini B, Pileri S, Zinzani PL, Carbone A, Zagonel V, Wolf- Peeters C, et al.ALK + lymphoma: clinical -pathological findings and outcome. Blood. 1999; 93(8): 2697- 2706
- Fraga M, Brousset P, Schlaifer D, Payen C, Robert A, Rubie H, et al. Bone marrow involvement in anaplastic large cell lymphoma. Immunohistochemical detection of minimal disease and its prognostic significance. Am J Clin Pathol. 1995; 103(1): 82- 8 9
- Mussolin L, Pillon M, d'Amore ES, Santoro N, Lombardi A, Fagioli F, et al. Prevalence and clinical implications of bone marrow involvement in pediatric anaplastic large cell lymphoma. Leukemia. 2005; 19 (9): 1643-1647
- Brugières L1, Le Deley MC, Rosolen A, Williams D, Horibe K, Wrobel G, et al. Impact of the methotrexate administration dose on the need for intrathecal treatment in children and adolescents with anaplastic large-cell lymphoma: results of a randomized trial of the EICNHL Group. J Clin Oncol. 2009; 27(6): 897-903.
- Lamant L, Pileri S, Sabattini E, Brugières L, Jaffe ES, Delsol G. Cutaneous presentation of ALK-positive anaplastic large cell lymphoma following insect bites: evidence for an association in five cases. Haematologica. 2010; 95(3): 449- 455.
- Benharroch D, Meguerian-Bedoyan Z, Lamant L, Amin C, Brugières L, Terrier-Lacombe MJ, et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood. 1998; 91(6): 2076- 2084.
- Vassallo J, Lamant L, Brugieres L, Gaillard F, Campo E, Brousset P, Delsol G. ALK-positive anaplastic large cell lymphoma mimicking nodular sclerosis Hodgkin's lymphoma: report of 10 cases. Am J Surg Pathol. 2006; 30(2): 223- 229.
- Falini B, Bigerna B, Fizzotti M, Pulford K, Pileri SA, Delsol G et al. ALK expression defines a distinct group of T / null lymphomas ("ALK lymphomas") with a wide morphological spectrum. Am J Pathol. 1998; 153(3): 875-886.
- Pileri SA, Pulford K, Mori S, Mason DY, Sabattini E, Roncador G, et al. Frequent expression of the NPM-ALK chimeric fusion protein in anaplastic large-cell lymphoma, lympho-histiocytic type. Am J Pathol. 1997; 150(4): 1207.
- Kinney MC, Collins RD, Greer JP, Whitlock JA, Sioutos N, Kadin ME. A small-cell-predominant variant of primary Ki-1 (CD30) + T-cell lymphoma. Am J Surg Pathol. 1993; 17(9): 859- 8 68.
- Cheuk W, Hill RW, Bacchi C, Dias MA, Chan JK. (2000) Hypocellular anaplastic large cell lymphoma mimicking inflammatory lesions of lymph nodes. Am J Surg Pathol. 2000; 24(11): 1537-1543.
- Montes- Mojarro IA, Steinhilber J, Bonzheim I, Quintanilla-Martinez L, Fend F. The Pathological Spectrum of Systemic Anaplastic Large Cell Lymphoma (ALCL). Cancers (Basel). 2018; 10(4): pii: E107.
- Stein H, Mason DY, Gerdes J, O'Connor N, Wainscoat J, Pallesen G, et al. The expression of the Hodgkin's disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985; 66(4): 848-858.
- Lamant L, Meggetto F, al Saati T, Brugières L, de Paillerets BB, Dastugue N, et al. High incidence of the t (2; 5) (p23; q35) translocation in anaplastic large cell lymphoma and its lack of detection in Hodgkin's disease. Comparison of cytogenetic analysis, reverse transcriptase-polymerase chain reaction, and P-80 immunostaining. Blood. 1996; 87(1): 284-291.
- Bonzheim I, Geissinger E, Roth S, Zettl A, Marx A, Rosenwald A, et al. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood. 2004; 104(10): 3358- 33 60.
- Delsol G, Al Saati T, Gatter KC, Gerdes J, Schwarting R, Caveriviere P, et al. Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol. 1988; 130(1): 59-70.
- Krenacs L, Wellmann A, Sorbara L, Himmelmann AW, Bagdi E, Jaffe ES, Raffeld M. Cytotoxic cell antigen expression in anaplastic large cell lymphomas of T- and null-cell type and Hodgkin's disease: evidence for distinct cellular origin. Blood. 1997; 89(3): 980- 989.
- Brugières L, Deley MC, Pacquement H, Meguerian-Bedoyan Z, Terrier-Lacombe MJ, Robert A, et al. CD30 (+) anaplastic large-cell lymphoma in children: analysis of 82 patients enrolled in two consecutive studies of the French Society of Pediatric Oncology. Blood. 1998; 92(10): 3591- 3598.
- Le Deley MC, Reiter A, Williams D, Delsol G, Oschlies I, McCarthy K, Zimmermann M, Brugières L, et al. European Intergroup for Childhood Non-Hodgkin Lymphoma. Prognostic factors in childhood anaplastic large cell lymphoma: results of a large European intergroup study. Blood. 2008; 111(3): 1560- 1566.
- Pasqualini C1, Minard -Colin V, Saada V, Lamant L, Delsol G, Patte C, et al. Clinical analysis and prognostic significance of haemophagocytic lymphohistiocytosis -associated anaplastic large cell lymphoma in children. Br J Haematol. 2014; 165(1): 117-125.
- Lowe EJ, Gross TG. Anaplastic large cell lymphoma in children and adolescents. Pediatr Hematol Oncol. 2013; 30 (6): 509-519.

