Case Report - Volume 3 - Issue 5
Delayed onset Takotsubo cardiomyopathy following post mitral valve placement
Thai Kieu Ngoc1; Sam Trevena2; Rebecca C. Olsen3; Anh Tien Hoang4; Viet Tang Thai5; Hoang Thi Nam Giang6; Minh Hung Ngo5; Huu Tu Le7; Nghi Viet Tran8; Ngoc Hien Nguyen5; Van Tuong Nguyen1; Phillip Tran5,9
1Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam.
2Midwestern University Arizona College of Osteopathic Medicine.
3A.T. Still University School of Osteopathic Medicine in Arizona, Phoenix, AZ.
4Internal Medicine Department, Hue University of Medicine and Pharmacy, Hue University Vice Director of Cardiovascular Centre, Hospital of Hue University of Medicine and Pharmacy – Hue Province, Vietnam.
5Nam Can Tho University, Can Tho City, Vietnam.
6School of Medicine and Pharmacy, The University of Danang, Danang City, Vietnam.
7Hue University of Medicine and Pharmacy, Hue Province, Vietnam.
8Weiss Memorial Hospital, Chicago, IL, USA.
9Cardiovascular Department, Yavapai Regional Medical Center, Prescott, AZ, Vietnam.
Received Date : Sep 25, 2023
Accepted Date : Oct 12, 2023
Published Date: Oct 19, 2023
Copyright: © Phillip Tran 2023
*Corresponding Author : Phillip Tran, Cardiovascular Department,
Yavapai Regional Medical Center, Prescott, AZ, Vietnem.
Email: PTranNYIT@gmail.com
DOI: Doi.org/10.55920/2771-019X/1571
Introduction
Takotsubo cardiomyopathy (TCM), also known as acute stress-induced cardiomyopathy or broken heart syndrome, is a temporary heart condition characterized by transient dysfunction and ballooning of the left ventricle (LV) of the heart. The condition is often attributed to events that cause major physical or emotional stress. Surgery may also impose substantial physiological and psychological stress on patients [1]. Diagnosis of TCM is typically based on the Mayo Clinic criteria and the criteria established by Kawai et al [2]. Only a few occurrences of TCM that have been preceded by cardiac surgery have been published, accounting for 0.9% according to 52 out of 5773 patients. In these cases, mitral valve surgery contributed to the majority of cases with 69.2% [3]. TCM typically occurs within 24 hours after surgery, accounting for 68% of cases [4]. We present a case of delayed TCM development four weeks after porcine mitral valve replacement due to severe mitral valve regurgitation.
Case presentation
An 84-year-old female with normal coronary arteries, as confirmed by a left heart catheterization (LHC) conducted two months prior, was initially admitted for porcine mitral valve replacement due to severe mitral regurgitation. On post-operative day 4, she developed sick sinus syndrome, necessitating the placement of a permanent pacemaker. Subsequently, on postoperative day 8, she experienced atrial fibrillation, which required cardioversion.
The patient was doing well until one month after the operation when she suddenly experienced chest pain, dizziness, and worsening dyspnea on exertion. She endorsed mild fatigue and weakness but denied any notable weight gain, lower extremity edema, or paroxysmal nocturnal dyspnea. Upon arrival at the emergency department, her vitals were stable. Cardiac examination was negative except for a grade 2/6 systolic murmur best heard at the apex and trace edema of the lower extremities. The remainder of the exam was unremarkable.
Initial laboratory results revealed mild leukocytosis, an elevated B-type natriuretic peptide (BNP) (286 pg/mL), and an initial troponin level of 48 ng/mL which rose to 176 ng/mL after two hours. An electrocardiogram was performed revealing the presence of a ventricular pacemaker (Fig 1).
A bedside echocardiogram was performed, revealing significant impairment of the left ventricle. The left ventricle ejection fraction was 25-30% and moderate aortic regurgitation and dilation of the left atrium was noted. The prosthetic mitral valve (surgically placed one month prior) was visualized without regurgitation and the mean diastolic gradient was measured as 3 mmHg. Interestingly, there was akinesis of the apical and mid-ventricular segments of the left ventricle, hypercontractility of the basal segments, and dilation of the apex (Fig 2, 3). Based on these findings, the patient was diagnosed with TCM and admitted for inpatient treatment. She was started on goal-directed medical therapy with empagliflozin 10 mg daily, lisinopril 2.5 mg daily, and metoprolol tartrate which was later changed to metoprolol succinate upon discharge. The patient was euvolemic during her hospital course so the addition of diuretics was not needed. Once her condition improved, she was discharged with a wearable defibrillator and scheduled for a follow-up with cardiology.
Discussion
TCM is a reversible cardiac impairment that predominantly affects post-menopausal women and patients with neurologic injuries [1]. Although the pathogenesis remains unclear, the condition is thought to be related to high catecholamine levels leading to sympathetic stimulation of the myocardium and cardiotoxicity [1]. Other hypotheses attribute the condition to microvascular dysfunction of the coronary arteries and/or abnormalities of cardiac fatty acid metabolism may play a role in the disease development [2].
Although previously a rare condition, there has been a notable increase in TCM cases over the last two decades with more than 1000 cases documented in the literature [2]. The incidence of TCM was estimated to be 1.5-1.8%, but there was a significant increase during the COVID-19 pandemic (March 1-April 30, 2020), 7.8% (5). Proposed triggers for this condition include sources of major emotional and/or physical stress. Minor stress is rarely associated with the onset of TCM and an estimated 30% of cases are idiopathic [6]. Examples of major stress include but are not limited to an accidental overdose of epinephrine, an adrenaline-producing tumor such as pheochromocytoma, surgery, the death of a loved one, domestic abuse, or natural disasters [7].
TCM typically manifests within 24 hours after surgery [4]. The case presented is unusual as TCM was diagnosed after undergoing mitral valve replacement, demonstrated by a gradual decline in cardiac function. In the literature, only two known cases of TCM have been diagnosed within 2-weeks of a surgical procedure [8, 9]. There are several reasons why invasive procedures may contribute to TCM as the anticipation and act of surgery are both emotionally and physically taxing. The withdrawal of anesthesia and shifts in intravascular fluid volume can also be sources of stress [4]. Prolonged operative times such as in the setting of a cardiac valve replacement are particularly stressful to the body as large amounts of catecholamines are released [4]. Additionally, mitral valve pathology is a well-established risk factor for TCM [3] but is unlikely to be the sole cause given the delayed onset of symptoms in this case. Other postoperative events in the presented case such as autonomic dysfunction during or after cardioversion, atrial fibrillation, sick sinus syndrome, and pacemaker placement [10, 11], may have played a role in the delayed onset of TCM during this timeframe.
It is estimated that 96% of TCM cases have a favorable prognosis and achieve a full recovery [12]. TCM, however, is not entirely benign. In a study of 136 patients, approximately 5% progressed to cardiac arrest or death despite appropriate aggressive treatment [13]. Complications are similar to acute MI and include acute heart failure, cardiogenic shock, acute kidney injury, ventricular and papillary muscle rupture, tamponade, and stroke. When compared to acute MI, TCM patients had higher rates of acute HF and stroke, but lower rates of other complications and in-hospital mortality [14].
Treatment for stress-induced cardiomyopathy is supportive and despite the complications above, the condition usually resolves on its own within several weeks. Hemodynamics may be stabilized with diuretics, angiotensin-converting enzyme inhibitors, and/or beta-blocker medications. Anticoagulation therapy should be initiated until the systolic function is recovered, unless there is a definite contraindication.
According to a study of 131 perioperative TCM cases, about 58% of patients experienced postoperative TCM. Of those postoperative cases, 63% occurred within the first 24 hours while 8% happened more than a week later [4]. There have been several theories regarding the association between TCM and atrioventricular valvular procedures (80% mitral, 11% tricuspid). Sudden correction of atrioventricular regurgitation causes abrupt changes in ventricular loading conditions which leads to an increase in endogenous catecholamines. Changes in protein expression, sarcomere arrangement, and myocyte contractility may play a role in causing the enlargement of the ventricle and atrium. In the setting of chronic severe atrioventricular repair, the sudden drop in ventricular preload red increase in afterload leads to a mismatch in myocardial mechanics, an increase in ventricular myocardial stress, and a transient increase in myocardial oxygen consumption. Finally, inflammation may also contribute to the development of this condition [3].
In a 5-year study of over 95,000 patients undergoing surgical procedures, the prevalence of post-operative TCM was 17.74 per 100,000 patients with a mortality rate of 23.5% [1]. This study identified three major risk factors for postoperative TCM development in adult patients: preoperative hyponatremia, obesity, and the American Society of Anesthesiologists (ASA) class 3 or greater [1]. ASA is a physical status classification system used to predict operative risk. Grade 3 refers to a patient with severe, non-life-threatening systemic disease such as morbid obesity, an implanted pacemaker, or poorly treated diabetes mellitus [15]. Grades 4 and above are used to describe life-threatening disease conditions [15]. In the presented case, the patient was a postmenopausal female with a preoperative BMI of 40 and a borderline-low sodium level of 135 mEq/l. These additional risk factors could have contributed to the development of this syndrome.
In conclusion, the initiation of open heart surgery, along with several stressful postoperative events and other identified risk factors, may collectively increase the likelihood of developing TCM in this patient.
Conclusion
Open heart surgery can act as a physical stressor that triggers TCM. TCM should be considered who developed acute LV dysfunction, even several weeks post-procedure. Early recognition of this condition is crucial for appropriate management. Supportive therapy plays a key role in the mainstay management of TCM.

Figure 1: EKG showed ventricular pacemaker.
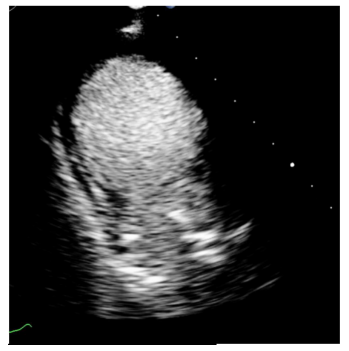
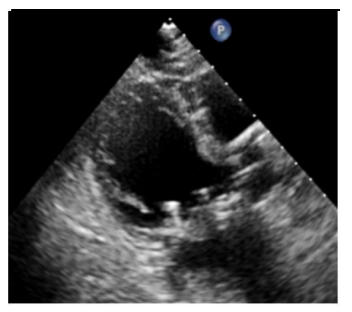
Figure 2, 3: Echocardiogram suggested Takotsubo Cardiomyopathy.
References
- Komamura K. Takotsubo cardiomyopathy: Pathophysiology, diagnosis and treatment. World Journal of Cardiology. 2014; 6(7): 602. https://doi.org/10.4330/wjc.v6.i7.602
- Tak Kyu Oh, In Ae Song, Young Mi Park, Jung Won Hwang, Young Tae Jeon, Hwan, S, et al. Prevalence and risk factors for postoperative stress-related cardiomyopathy in adults. PLOS ONE. 2017; 12(12): e0190065-e0190065. https://doi.org/10.1371/journal.pone.0190065
- Laghlam D, Touboul O, Herry M, Estagnasié P, Dib JC, Baccouche M, et al. Takotsubo cardiomyopathy after cardiac surgery: A case-series and systematic review of literature. Frontiers in Cardiovascular Medicine. 2023; 9: 1067444.
- Hessel EA. Takotsubo cardiomyopathy and its relevance to anesthesiology: a narrative review. Canadian Journal of Anesthesia/Journal Canadien D’anesthésie. 2016; 63(9): 1059-1074. https://doi.org/10.1007/s12630-016-0680-4
- Jabri A, Kalra A, Kumar A, Alameh A, Adroja S, Bashir H, et al. Incidence of Stress Cardiomyopathy during the Coronavirus Disease 2019 Pandemic. JAMA Network Open. 2020; 3(7): e2014780-e2014780. https://doi.org/10.1001/jamanetworkopen.2020.14780
- Gherasim L. Takotsubo Syndrome versus Neurogenic Stunned Myocardium. Maedica. 2020; 15(3): 288-296. https://doi.org/10.26574/maedica.2020.15.3.288
- Articles. (n.d.). Cedars-Sinai. https://www.cedars-sinai.org/health-library/diseases-and-conditions/t/takotsubo-cardiomyopathy.html
- Färber G, Mühle A, Doenst T, Borger M, Mohr FW. Late Onset Takotsubo Cardiomyopathy after Mitral Valve Replacement. The Thoracic and Cardiovascular Surgeon. 2011; 59(08): 500-503. https://doi.org/10.1055/s-0030-1270798
- Lorca R, Callejo F, Pun F, Martín M, Corros C, Alperi A, ert al. Takotsubo syndrome after heart valve surgery. International Journal of Cardiology. 2015; 197: 254-256.
- Agarwal S, Sanghvi C, Odo N, Castresana MR. Perioperative Takotsubo Cardiomyopathy: Implications for Anesthesiologist. Annals of Cardiac Anaesthesia. 2019; 22(3): 309-315. https://doi.org/10.4103/aca.ACA_71_18
- 김준. Takotsubo cardiomyopathy complicating sick sinus syndrome. International Journal of Arrhythmia. 2013; 14(2): 24-28.
- Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-Year Recurrence Rate and Prognosis of the Apical Ballooning Syndrome. Journal of the American College of Cardiology. 2007; 50(5): 448-452. https://doi.org/10.1016/j.jacc.2007.03.050
- Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, et al. Natural History and Expansive Clinical Profile of Stress (Tako-Tsubo) Cardiomyopathy. Journal of the American College of Cardiology. 2010; 55(4): 333-341. https://doi.org/10.1016/j.jacc.2009.08.057
- Vallabhajosyula S, Barsness GW, Herrmann J, Anavekar NS, Gulati R, Prasad A. Comparison of Complications and In-Hospital Mortality in Takotsubo (Apical Ballooning/Stress) Cardiomyopathy Versus Acute Myocardial Infarction. The American Journal of Cardiology. 2020; 132: 29-35. https://doi.org/10.1016/j.amjcard.2020.07.015
- Daniel John Doyle, Garmon EH. American Society of Anesthesiologists Classification (ASA Class). Nih.gov; StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK441940/. 2019.

