Case Report and Comprehensive Review - Volume 3 - Issue 5
From chest pain to dual diagnosis: navigating the nuances of Takotsubo cardiomyopathy and acute pericarditis
Huu Tu Le1; Hoang Duc Nguyen2; Neha Prakash3; Anh Tien Hoang4; Minh Hung Ngo5; Anh Binh Ho6; Anh Tu Hoang5; Damon Huynh7; Van Tuong Nguyen8; Phillip Tran5,9*
1Hue University of Medicine and Pharmacy, Hue Province, Vietnam.
2Methodist Hospital Merrillville, Indiana.
3A.T. Still University, AZ, Vietnam.
4Internal Medicine Department, Hue University of Medicine and Pharmacy, Hue University Vice Director of Cardiovascular Centre, Hospital of Hue University of Medicine and Pharmacy – Hue Province, Vietnam.
5Nam Can Tho University, Can Tho City, Vietnam.
6Cardiovascular center, Hue Central Hospital, Vietnam.
7Sea Mar Family Medicine, Vietnam.
8Pham Ngoc Thach University of Medicine, Ho Chi Minh City, Vietnam.
9Cardiovascular Department, Yavapai Regional Medical Center, Prescott, AZ, Vietnam.
Received Date : Sep 27, 2023
Accepted Date : Oct 16, 2023
Published Date: Oct 23, 2023
Copyright: © Phillip Tran 2023
*Corresponding Author : Phillip Tran, Cardiovascular Department,
Yavapai Regional Medical Center, Prescott, AZ, Vietnem. Tel: (515) 473-
0727
Email: PTranNYIT@gmail.com
DOI: Doi.org/10.55920/2771-019X/1572
Abstract
Background: Takotsubo cardiomyopathy and acute pericarditis are distinct cardiac entities. Their simultaneous occurrence is rare.
Case Presentation: A 70-year-old female experienced sudden chest pain with radiating symptoms, nausea, and near-syncope. Initial EKG showed ST-elevation, later transitioning to T wave inversions. Despite elevated troponin, coronary arteries were non-obstructed. Echocardiography revealed a reduced left ventricular ejection fraction with apex akinesis. These findings led to a dual diagnosis of Takotsubo cardiomyopathy and acute pericarditis. Treatment with aspirin, lisinopril, and metoprolol yielded notable improvement by the one-month follow-up.
Discussion: The coexistence of these conditions challenge diagnostic and treatment paradigms. Emotional stress might have triggered the onset, with EKG changes and non-recurrence of pericarditis underscoring the case's uniqueness.
Conclusion: This case highlights the importance of considering rare coexisting cardiac conditions and emphasizes tailored treatments for nuanced presentations.
Keywords: Takotsubo cardiomyopathy, Acute pericarditis, Echocardiography, ST-elevation, Cardiology, Case report
Introduction
Takotsubo cardiomyopathy, also commonly known as stress-induced cardiomyopathy or 'broken heart syndrome,' was first described by Japanese physicians in the 1990s and has since garnered global recognition [1]. It is characterized by transient regional systolic dysfunction, typically affecting the apex of the left ventricle, and often mimics the clinical presentation of acute coronary syndrome. However, a key distinguishing feature of this condition is the absence of obstructive coronary artery disease on angiography [2]. While pericarditis associated with Takotsubo is rare, it can manifest in different ways. This case report describes a patient who presented with chest pain and elevated troponin levels, ultimately diagnosed with both Takotsubo cardiomyopathy and acute pericarditis.
Case presentation
A 70-year-old woman sought emergency care after experiencing sudden, intense chest pain that seemed to radiate to her back and left arm. Accompanying this distress were sensations of nausea and episodes of near-syncope. At presentation, her vital signs remained stable — blood pressure, heart rate, respiratory rate, and temperature were all within normal parameters. The physical examination was largely non-contributory, showing no obvious signs that might pinpoint the cause of her discomfort.
To further evaluate the cause of her symptoms, an electrocardiogram (EKG) was performed, revealing prominent ST-elevation in the inferior and lateral leads, suggestive of a myocardial event (Figure 1). This pattern was further complicated by the presence of multiple premature ventricular contractions (PVCs). A mere day later, a second EKG showed a shift in the cardiac pattern, now displaying widespread T wave inversions (Figure 2). Complementing these findings, her blood tests returned with markedly elevated high-sensitivity troponin levels at 4561 ng/mL, an indicator of cardiac muscle injury. An immediate left heart catheterization was undertaken to evaluate the patency of her coronary arteries. The results were unexpected, showing non-obstructed coronary arteries (Figure 3). An echocardiogram provided additional insight into the cardiac function, demonstrating a reduced left ventricular ejection fraction of 35-40%. The imaging also revealed normal contractility in most segments but showed mild segment hypokinesis and a complete akinesis of the apex (Figure 4).
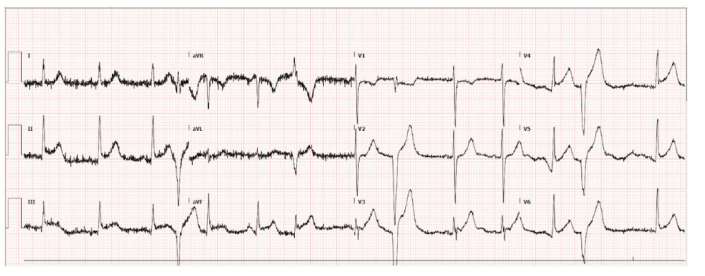
Figure 1: EKG shows diffuse ST elevations in lead II, III, and V2 to V6.
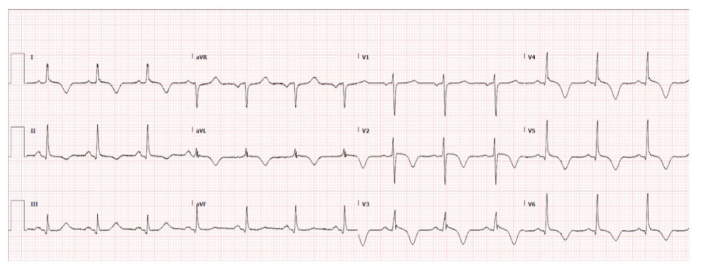
Figure 2: EKG shows widespread T wave inversion.
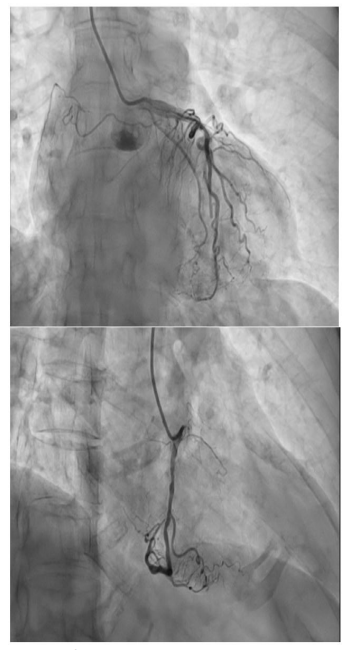
Figure 3: Left heart catheterization shows no coronary arterial obstruction.
Taking into account all the clinical and investigative findings, a diagnosis of stress-induced cardiomyopathy was reached. However, given the presentation and EKG findings, there was also an evident concurrent episode of acute pericarditis. For her management, she was started on a medical regimen that included aspirin (81 mg daily), lisinopril (5 mg daily), and metoprolol (12.5 mg daily). The effectiveness of this therapeutic approach became evident at her one-month follow-up, where she reported no recurrence of chest pain. Interestingly, she never commenced on colchicine therapy, a common treatment for pericarditis. This positive clinical trajectory was further substantiated at her five-month follow-up. An echocardiogram at that time displayed a fully recovered left ventricular ejection fraction and no discernible signs of pericardial effusion or thickening.
Discussion
Within the intricate landscape of cardiology, our patient's simultaneous presentation of Takotsubo cardiomyopathy and acute pericarditis offers a unique clinical perspective. The echocardiographic findings, showcasing a notable improvement in the left ventricular ejection fraction, paired with the absence of coronary artery abnormalities from left heart catheterization, gently align with the diagnostic criteria set by the Mayo Clinic for Takotsubo cardiomyopathy [3]. Yet, what adds complexity to this case is the coexistence of acute pericarditis, subtly hinted by the evolving ECG changes. The shift from diffuse ST elevation to a more pronounced T wave inversion is a noteworthy observation. Such a confluence is a rarity, with minimal cases reported in the literature that delve into this overlapping clinical spectrum.
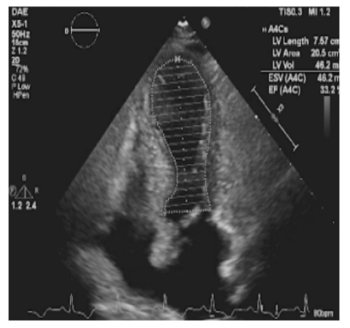
Figure 4: Echocardiography shows apical ballooning of the left ventricle.
The exact cause of Takotsubo cardiomyopathy isn't fully known. Possible factors include: sympathetic overdrive, coronary spasm, microvascular dysfunction, low estrogen, inflammation, or impaired fatty acid metabolism [2, 3]. Takotsubo cardiomyopathy, frequently associated with emotional and physical stressors, can lead to sympathetic overdrive. Our patient's intense emotional stress episode might have served as a triggering event. The subsequent development of acute pericarditis could potentially arise from the transmural inflammation observed in Takotsubo cardiomyopathy, affecting the adjoining pericardium [4]. Conversely, it's possible that the severe chest pain from pericarditis could trigger a catecholamine surge, predisposing the heart to Takotsubo [5]. Moreover, prior study on murine models by Wu et al. showed that rising catecholamine can induce cardiomyocyte necroptosis that contributes to myocardial injuries [6]. Furthermore, Wang et al. reported a case with fatal pheochromocytoma showing catecholamine-related cardiotoxicity [7]. Noteworthy in our patient’s narrative is the temporal proximity of the two conditions, sans any underlying autoimmune pathology, but intriguingly aligned with an episode of pronounced emotional stress. This intricate chronology leads us to postulate that the onset of Takotsubo cardiomyopathy perhaps paved the way for pericarditis, a conjecture strengthened by the ECG progression. The subsequent resolution of pericarditis, without the intervention of colchicine and no recurrence over a year, further accentuates the distinctiveness of our patient’s clinical journey.
The diagnostic trajectory for our patient emphasized the challenges in differentiating between overlapping cardiac conditions. Utilizing the Mayo Clinic criteria, we identified the transient ventricular abnormalities, ECG changes, and absence of obstructive coronary disease suggestive of Takotsubo cardiomyopathy [3]. The rapid progression from ST elevation to T-wave inversion on the patient's ECG, paired with other clinical signs, was consistent with acute pericarditis. Such concurrent diagnoses emphasize the need for thorough, discerning evaluations in cardiac presentations that might mask as more common entities.
In managing such a multifaceted clinical picture, treatment strategies need to be adaptable. Management of Takotsubo cardiomyopathy lacks definitive guidelines, relying on clinical experience and expert consensus (evidence level C) [8]. Initial treatment mirrors acute coronary syndrome with aspirin, beta-blockers, ACE inhibitors, lipid-lowering agents for 3-6 months, in addition to coronary angiography to rule out artery disease. Anticoagulation is reserved for ventricular thrombus or embolic events [8]. In our patient, the presence of acute chest pain, elevated troponin levels, and ECG changes implied a cardiac event of significant concern. The immediate priority was to rule out obstructive coronary artery disease, thus, an urgent coronary angiography was performed, confirming the absence of such obstruction and pointing toward Takotsubo. With acute pericarditis also on the differential—given the ECG changes—the decision was made to treat both simultaneously. We chose a treatment plan involving aspirin, lisinopril, and metoprolol, covering the primary therapeutic recommendations for both conditions. The patient's rapid improvement, particularly in the left ventricular ejection fraction, further reinforced our treatment approach.
Comparing our case with other reported cases in the literature highlights some different aspects. For example, Kim et al. reported a similar case with both Takotsubo and pericarditis [9]. However, while both displayed ST-segment elevations, the evolution of these changes differed. Kim et al. employed colchicine, resulting in normalized EKG changes and ejection fraction within four weeks [9]. Mori et al. reported a rheumatoid arthritis patient with Takotsubo complicated by pericarditis.10 In comparison to the 64-year-old woman reported by Mori et al., our patient also demonstrated apical ballooning and pericardial effusion. However, the 64-year-old exhibited more distinct ECG changes, including elevated ST-segments and inverted T-waves, alongside an incomplete right bundle-branch block. Regarding the choice of therapy, Mori et al. treated the patient pericarditis and RA concomitantly with corticosteroid and anticoagulants [10]. Our approach, meanwhile, adopted a broader therapeutic strategy, highlighting the nuanced nature of managing such cases.
Conclusion
The confluence of acute pericarditis and Takotsubo cardiomyopathy is a seldom-encountered scenario in clinical practice, making its documentation crucial for the broader medical community. Our case not only highlights the importance of recognizing this rare association but also delves into potential complications, such as the risk of cardiac tamponade. As clinicians navigate the intricacies of managing these intertwined conditions, it becomes imperative to reconsider standard treatments, including the customary three-month-duration use of colchicine. Interestingly, when Takotsubo cardiomyopathy coexists with pericarditis, the latter often exhibits a transient nature with a lower propensity for recurrence, prompting the need for a more tailored therapeutic approach.
References
- Kida K, Akashi YJ, Fazio G, Novo S. Takotsubo cardiomyopathy. Curr. Pharm. Des. 2010; 16, 2910-2917.
- Assad J, Femia G, Pender P, Badie T, Rajaratnam R. Takotsubo Syndrome: A Review of Presentation, Diagnosis and Management. Clin. Med. Insights Cardiol. 2022; 16: 11795468211065782.
- Boyd B, Solh T. Takotsubo cardiomyopathy: Review of broken heart syndrome. JAAPA. 2020; 33: 24-29.
- Khandaker MH, et al. Pericardial disease: diagnosis and management. Mayo Clin. Proc. 2010; 85: 572-593.
- Casey RT, et al. Management of an acute catecholamine-induced cardiomyopathy and circulatory collapse: a multidisciplinary approach. Endocrinol. Diabetes Metab. Case Rep. 2017; 2017: 17-0122.
- Wu P, Cai M, Liu J, Wang X. Catecholamine Surges Cause Cardiomyocyte Necroptosis via a RIPK1-RIPK3-Dependent Pathway in Mice. Front. Cardiovasc. Med. 2021; 8: 740839.
- Wang AR, Dean SA, Grebe SK, Hood IC. Fatal Catecholamine-Induced Cardiotoxicity Associated with Pheochromocytoma: Report of a Postpartum Case and Review of the Literature. Acad. Forensic Pathol. 2016; 6: 315-324.
- Ghadri JR,et al. International Expert Consensus Document on Takotsubo Syndrome (Part II): Diagnostic Workup, Outcome, and Management. Eur. Heart J. 2018; 39: 2047-2062.
- Kim J, Laird-Fick HS, Alsara O, Gourineni V, Abela GS. Pericarditis in Takotsubo Cardiomyopathy: A Case Report and Review of the Literature. Case Rep. Cardiol. 2013; 2013: 1-5.
- Mori K, Yagi M, Oe K, Shimojima M, Yamagishi M. Pericarditis-complicated takotsubo cardiomyopathy in a patient with rheumatoid arthritis. Cardiovasc. Diagn. Ther. 2018; 8: 520-524.

