Case report - Volume 3 - Issue 6
Ibrutinib-induced cystoid Macular oedema
Mahmoud Eissa*; Christina Mathew; Rashi Arora
Department of Ophthalmology, Salisbury District Hospital, Odstock Rd, Salisbury SP2 8BJ. United Kingdom.
Received Date : Oct 09, 2023
Accepted Date : Nov 03, 2023
Published Date: Nov 10, 2023
Copyright:© Mahmoud Eissa 2023
*Corresponding Author : Mahmoud Eissa, Department of Ophthalmology, Salisbury District Hospital, Odstock Rd, Salisbury SP2 8BJ. United Kingdom.
Email: Mahmoud.eissa@nhs.net
DOI: Doi.org/10.55920/2771-019X/1580
Abstract
Ibrutinib is a small-molecule drug approved for the treatment of mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenstrom macroglobulinemia. Although it has been connected to ocular side effects, few clinical cases explain cystoid macular oedema associated with long-term Ibrutinib treatment. A case of Cystoid Macular Oedema (CMO) caused by ibrutinib in a patient with chronic lymphocytic leukemia is presented. In our case study, an 85-year-old man’s vision deteriorated over 6-7 months. His right eye’s presenting vision was 0.76 and his left eye was 0.2. There was bilateral macular oedema on inspection. His ibrutinib treatment lasted 12 months. The temporal correlation between ibrutinib treatment modifications and the ocular inflammation in our patient points to a causal relationship. Ibrutinib is thought to cause drug-induced cystoid macular oedema. The chronological association between ibrutinib treatment adjustments, or other Tyrosine Kinase inhibitors and our patient’s macular oedema suggests a causal relationship.
Introduction
Tyrosine kinase is an important proto-oncogene that is vital in the cascade of B-cell maturation and plays a role in the stimulation of epidermal growth factor receptors helping in motility, proliferation, and survival of normal and malignant B-Cell in addition to the synthesis of immunoglobulin [1]. Ibrutinib belongs to a group called tyrosine kinase inhibitors which irreversibly inhibits the tyrosine kinase pathway, thereby reducing B malignant cells in different types of malignancy. It has been approved for use in mantle cell lymphoma, chronic lymphocytic leukemia, and Waldenstrom macroglobulinemia [2]. The most common side effects of Ibrutinib are diarrhoea, upper respiratory tract infection, bleeding, fatigue, and cardiac side effects most commonly atrial fibrillation [3, 4]. While Ibrutinib has been reported to cause multiple ocular side effects such as red eye, dry eye, uveitis, branch retinal vein occlusion, and cystoid macular oedema, these reports are very few and far between [5]. Importantly this case demonstrated cystoid macular oedema with several tyrosine kinase inhibitors and highlights the necessity of collaborative working between physicians and ophthalmologists in managing these complications.
Case report
An 85-year-old male was referred by his optometrist to the macula clinic for deteriorating vision over the course of 6-7 months with a drop in the visual acuity in the right eye. His best corrected visual acuity was 0.76 and 0.2 in the right and left eye respectively. His past medical history was significant for chronic lymphocytic leukaemia for which he takes Ibrutinib for 1 year and the addition of co-trimoxazole 5 months before the onset of his symptoms. He had no other significant ocular or systemic medical history. On examination, he had bilateral non-significant nuclear cataract, epiretinal membrane, and bilateral macular oedema. Optical coherence tomography (Cirrus HD-OCT 6000-13769) confirmed bilateral macular oedema (Fig.1). Fundus fluorescein angiography (FFA) was organized 2 weeks later showing mild perifoveal leakage in the right eye perifoveal leakage in the right eye. There was no evidence of vasculitis (Fig.2). Following consultation with the haematologist, ibrutinib was stopped.
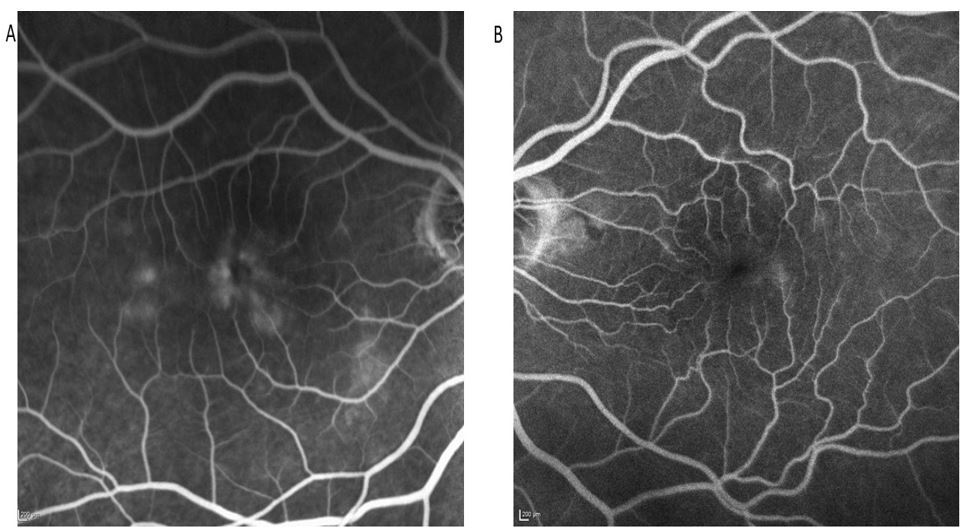
Figure 1: OCT showing bilateral cystoid macular oedema at the initial visit.
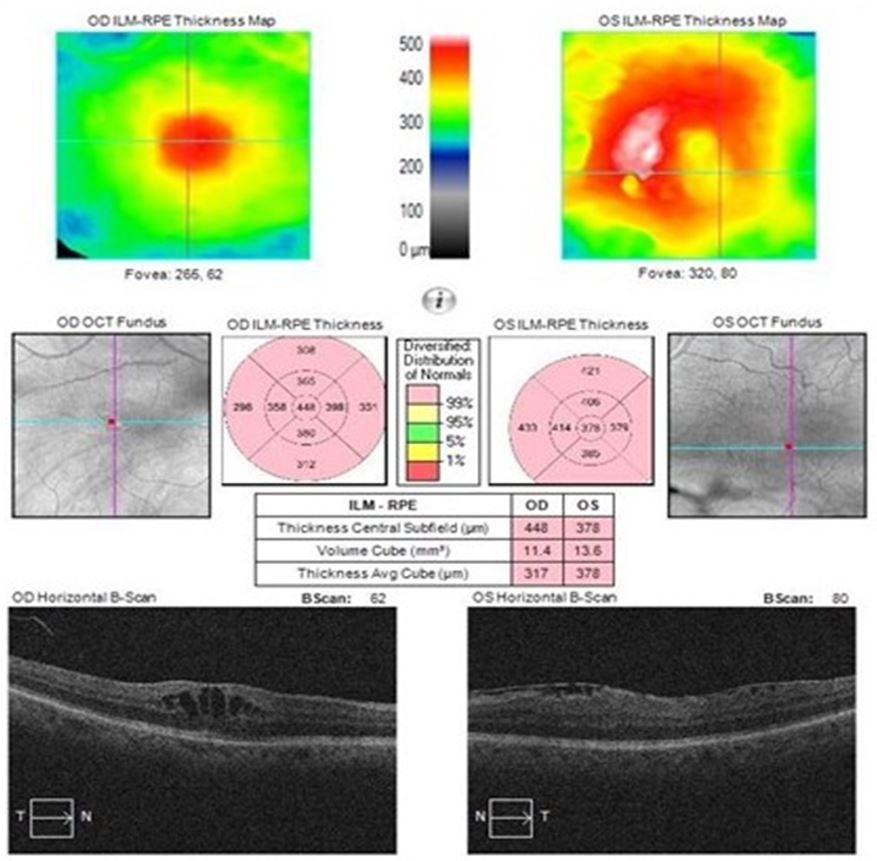
Figure 2: (a) RE FFA showing hyper-florescence leakage, (b) LE FFA showing no signs of leakage.
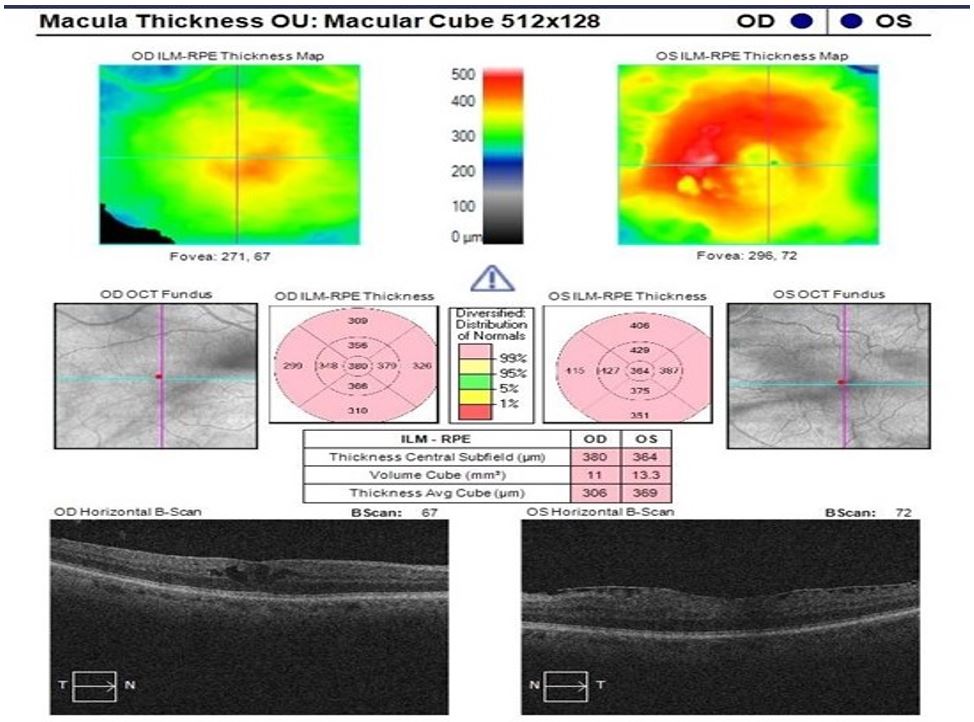
Figure 3: OCT shows a decrease in macular thickness and intraretinal fluid 4 weeks after cessation of Ibrutinib.
At a 4-week follow-up after stopping Ibrutinib, there was an improvement in the best corrected visual acuity in the right eye to 0.46 from 0.76. In addition, OCT indicated reduced intraretinal fluid (Fig.3). However, at an 8-week follow-up, while the visual acuity remained stable at 0.58 in the right eye, there was a recurrence of intraretinal fluid. On eliciting further history, the patient reported that he had been restarted on ibrutinib 3 weeks prior as he did not feel well while he was off the drug. After consulting with the haematology consultant, the Ibrutinib dose was reduced to half, and at his 8-week follow-up, the OCT showed a reduction in the fluid. The patient was scheduled to undergo right eye cataract surgery and ibrutinib was stopped 7 days prior. The operation was uneventful and at a routine 4-month follow-up, his BCVA improved to 0.42 in the right eye. Nonetheless, his OCT indicated an increase in intraretinal fluid. The patient reported that the ibrutinib was stopped and replaced by another tyrosine kinase inhibitor namely Zanubrutinib 80 mg OD. He was treated with topical steroids and anti-inflammatory drops for cystoid macular oedema (CMO). The drug was withdrawn.
The 4-week follow-up showed an improvement in visual acuity in the right eye to 0.38 and a decrease in fluid which continued to improve at a further 3-month follow-up. During his latest review in the Uveitis clinic, the CMO had increased in the right eye with a subjective decrease in vision and a slight drop in BCVA from 0.38 to 0.36 after Zanubrutinib was restarted. Since the patient requires lifelong tyrosine kinase inhibitor therapy, he continued to remain under our care to monitor the CMO.
Discussion
Macular oedema is one of the causes of gradual loss of visual acuity that if left untreated can lead to marked visual impairment and it can be caused by many factors like diabetes, hypertension, retinal vein occlusion, post-surgical, and uveitis [6-8]. Drug-induced macular oedema has been attributed to a number of drugs like Tamoxifen [9], nicotinic Acid [10], Paclitaxel and Bevacizumab [11], and ocular drugs such as prostaglandin and Timolol [12].
Ibrutinib has multiple known side effects which include more commonly GIT symptoms like diarrhoea (50%), nausea (26%), constipation (21%) and vomiting (20%), cough and fatigue (36%), peripheral oedema (27%) in addition to, anaemia, pyrexia, arthralgia and HTN and others [13]. They also cause ocular side effects such as dry eye due to ocular toxicity and red eye secondary to subconjunctival haemorrhage [14]. Uveal tract inflammation (uveitis) was also reported in multiple case reports [15, 16]. A study that evaluated the efficacy of Ibrutinib vs Ofatumumab found that 10% of patients on Ibrutinib developed blurred vision in addition to 3% who developed cataracts compared to 1% on Ofatumumab [17].
Potential posterior segment complications include branch retinal artery occlusion [18], and more recent case reports have indicated that ibrutinib produces drug induced macular oedema. The mechanism of action related to ibrutinib-induced cystoid macular oedema has not been found. There is a study suggesting Ibrutinib disrupts the blood-retinal barrier as it is known to cross the blood-brain barrier [19].
Response to steroids and anti-inflammatory topical therapy is mixed with some authors reporting only temporary resolution or minimal reduction of fluid with more complete resolution noted only after cessation of the drug entirely [20], while others have noted resolution of symptoms and fluid without the need to reduce the dose of the drug or stop the drug [19, 21]. Indeed, in this case, while reducing the dose of ibrutinib decreased intraretinal fluid, fluid resolved entirely only when the drug was completely withdrawn. Additionally, replacing Ibrutinib with Zanubrutinib caused a recurrence of symptoms and CMO. Therefore tyrosine kinase inhibitors might be associated with cystoid macular oedema.
Conclusion
Ibrutinib associated cystoid macular oedema is a recently documented ocular side effect and this report highlights the importance of ophthalmologists working collaboratively with haematologists and rheumatologists to monitor patients receiving not only Ibrutinib but also any tyrosine kinase inhibitors. Awareness of these ocular complications and proactive management are crucial to ensure timely intervention and preservation of visual function in patients undergoing Ibrutinib treatment. Consequently, it is crucial that doctors who often prescribe Tyrosine Kinase Inhibitors are aware of these potential side effects.
Recommendations: We would recommend that it becomes a standard practice for patients on tyrosine kinase inhibitors to be monitored by an eye specialist regularly or to offer patient self-monitoring tools like Amsler grid and to be aware if there are any eye symptoms to contact their health professionals for assessment.
References
- Roskoski R. Src protein-tyrosine kinase structure and regulation. Biochemical and Biophysical Research Communications. 2004; 324(4): 1155-1164. https://doi.org/10.1016/j.bbrc.2004.09.171.
- Wang ML, Blum KA, Martin P, Goy A, Auer R, Kahl BS, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: Updated safety and efficacy results. Blood. 2015; 126(6): 739-745. https://doi.org/10.1182/blood-2015-03-635326.
- Lasica M, Tam CS. Management of Ibrutinib Toxicities: A Practical Guide. Current Hematologic Malignancy Reports. 2020; 15(3): 177-186. https://doi.org/10.1007/s11899-020-00576-3.
- Paydas S. Management of adverse effects/toxicity of ibrutinib. Critical Reviews in Oncology/Hematology. 2019; 136: 56-63. https://doi.org/10.1016/j.critrevonc.2019.02.001.
- Chiu ZK, Goh JK, Ling C, Lin ML, Hall AJ. Ibrutinib-related uveitis: A case series. American Journal of Ophthalmology Case Reports. 2022; 25. https://doi.org/10.1016/j.ajoc.2022.101300.
- Daruich A, Matet A, Moulin A, Kowalczuk L, Nicolas M, Sellam A, et al. Mechanisms of macular edema: Beyond the surface. Progress in Retinal and Eye Research. 2018; 63: 20-68. https://doi.org/10.1016/j.preteyeres.2017.10.006.
- Fardeau C, Champion E, Massamba N, LeHoang P. Uveitic macular edema. Eye. 2016; 30(10): 1277. https://doi.org/10.1038/eye.2016.115.
- Lobo C. Pseudophakic Cystoid Macular Edema. Ophthalmologica. 2011; 227(2): 61-67. https://doi.org/10.1159/00033127.
- Zafeiropoulos P, Nanos P, Tsigkoulis E, Stefaniotou M. Bilateral Macular Edema in a Patient Treated with Tamoxifen: A Case Report and Review of the Literature. Case Reports in Ophthalmology. 2014; 5(3): 451-454. https://doi.org/10.1159/000370144.
- Domanico D, Carnevale C, Fragiotta S, Verboschi F, Altimari S, Vingolo EM. Cystoid Macular Edema Induced by Low Doses of Nicotinic Acid. Case Reports in Ophthalmological Medicine. 2013; 713061. https://doi.org/10.1155/2013/713061.
- Yokoe T, Fukada I, Kobayashi K, Shibayama T, Miyagi Y, Yoshida A, et al. Cystoid Macular Edema during Treatment with Paclitaxel and Bevacizumab in a Patient with Metastatic Breast Cancer: A Case Report and Literature Review. Case Reports in Oncology. 2017; 10(2): 605-612. https://doi.org/10.1159/000477897.
- Tang Y, Dou R, Liu Y, Xie S, Han Q. Loratadine-associated cystoid macular edema: A case report. American Journal of Ophthalmology Case Reports. 2022; 26: 101477. https://doi.org/10.1016/j.ajoc.2022.101477.
- Burger JA, Barr PM, Robak T, Owen C, Ghia, P, Tedeschi A, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020; 34(3): 787-798. https://doi.org/10.1038/s41375-019-0602-x.
- Farooqui MZ H, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: A phase 2, single-arm trial. The Lancet. Oncology. 2015; 16(2): 169-176. https://doi.org/10.1016/S1470-2045(14)71182-9.
- Arepalli S, Srivastava SK, Baynes K, Venkat AG. Panuveitis Presumed Secondary to Ibrutinib Therapy. Ophthalmic Surgery, Lasers & Imaging Retina. 2021; 52(3): 160-164. https://doi.org/10.3928/23258160-20210302-08.
- Bohn M, Bravo-Ljubetic L, Lee RWJ, Petrushkin H. Ibrutinib-related uveitis: A report of two severe cases. European Journal of Ophthalmology. 2022; 32(4): NP94-NP97. https://doi.org/10.1177/11206721211001268.
- Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. The New England Journal of Medicine. 2014; 371(3): 213-223. https://doi.org/10.1056/NEJMoa1400376.
- Kunkler AL, Binkley EM, Mantopoulos D, Hendershot AJ, Ohr MP, Kendra KL, et al. Known and novel ocular toxicities of biologics, targeted agents, and traditional chemotherapeutics. Graefe’s Archive for Clinical and Experimental Ophthalmology = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie. 2019; 257(8): 1771-1781. https://doi.org/10.1007/s00417-019-04337-8.
- Ben-Avi R, Dori,D, Chowers I. Cystoid macular edema secondary to ibrutinib. American Journal of Ophthalmology Case Reports. 2022; 26: 101436. https://doi.org/10.1016/j.ajoc.2022.101436.
- Mirgh SP, Ahmed R, Agrawal N, Bothra S, Mohan B, Garg A, et al. Knowing the Flip Side of the Coin: Ibrutinib Associated Cystoid Macular Edema. Indian Journal of Hematology & Blood Transfusion: An Official Journal of Indian Society of Hematology and Blood Transfusion. 2020; 36(1): 208-210. https://doi.org/10.1007/s12288-019-01181-y.
- Saenz-de-Viteri M, Cudrnak T. Bilateral cystoid macular edema in a patient with chronic lymphocytic leukemia treated with ibrutinib. Leukemia & Lymphoma. 2019; 60(3): 842-844. https://doi.org/10.1080/10428194.2018.1508673.

