Case report - Volume 3 - Issue 6
Cardiac tampoande following percutaneous coronary intervention of unknown etiology
Dražen Bedeković1,3*; Jerko Arambašić1; Ivica Bošnjak1,3; Damir Kirner1,3; Stefan Gjoni 2,3; Fabian Gjoni 2,3; Marija Glasnović 2,3
Department of Ophthalmology, Salisbury District Hospital, Odstock Rd, Salisbury SP2 8BJ. United Kingdom.
Received Date : Oct 10, 2023
Accepted Date : Nov 06, 2023
Published Date: Nov 13, 2023
Copyright:© Dražen Bedeković 2023
*Corresponding Author : Dražen Bedeković, MD, University hospital Osijek, Division of Cardiology, Osijek, Croatia.
Email: drbedekovic@yahoo.com
DOI: Doi.org/10.55920/2771-019X/1581
Abstract
Cardiac tamponade is a life-threatening condition characterized by the accumulation of fluid, blood, pus, clots, or even gas within the pericardium which compromise of cardiac function. It can occur due to various causes such as inflammation, trauma, heart rupture, aortic dissection. Recently it has become increasingly common as a complication of complex cardiac interventions usually caused by epicardial artery rupture. In this case, we present a patient who developed delayed cardiac tamponade following percutaneous coronary intervention, with no apparent cause identified despite extensive investigation using imaging methods. After antiplatelet therapy de-escalation pericardial bleeding in ceased and patient had survived with full recovery.
Introduction
Cardiac tamponade is a life-threatening condition characterized by the accumulation of fluid, blood, pus, clots, or even gas in the pericardium and heart compression with consequential loss of function. It can occur as a result of inflammation, trauma, heart rupture, aortic dissection, or as a complication of complex cardiac interventions [1].
Recent studies on cardiac tamponade have reported various causes, including percutaneous cardiac interventions (accounting for up to 36% of all cases), malignancies (up to 23%), infectious/inflammatory causes (up to 15%), and mechanical complications of myocardial infarction (up to 12%) [2].
The classic presentation of cardiac tamponade was initially described by the American thoracic surgeon Claude Schaeffer Beck in 1935. Beck identified a triad of symptoms, including hypotension, increased jugular venous pressure, and a small and quiet heart. These signs are typically observed in patients with rapid fluid accumulation in the pericardium, but they may be absent in cases of slower accumulation [3].
Echocardiography has become the most valuable diagnostic tool for detecting pericardial effusion, assessing its size, location, and hemodynamic impact. It plays a crucial role in identifying the underlying causes and provides important insights into general heart condition providing help in appropriate treatment [4].
Cardiac tamponade is primarily managed by echocardiography-guided pericardiocentesis, which is the preferred treatment of choice. Alternatively, fluoroscopic-guided pericardiocentesis can also be performed. However, unguided procedures are not recommended due to limited precision and risk of heart injury. In cases of purulent effusions, large fluid volumes, or challenging access to the fluid, surgical drainage is favored over percutaneous drainage. Surgical intervention provides better control and visualization, allowing for more effective management of complex or severe cases of cardiac tamponade [1].
Case report
We present a 65-year old patient who was admitted to the intensive cardiology unit due to chest pain experienced over the past five months, with an intensity increase during past month. The patient has a history of type 2 diabetes and arterial hypertension. He had a myocardial infarction 30 years ago and underwent coronary angiography, however, medical records regarding this procedure was not available.
Initially, an echocardiography was performed, which revealed a preserved ejection fraction of the left ventricle but showed mild hypokinesis of the lateral wall. The right ventricle exhibited preserved systolic function, and there were no significant abnormalities detected in the heart valves or signs of pericardial effusion. Subsequently, a coronary angiography was made revealing bifurcation sub-occlusive stenosis of the proximal segment of the left anterior descending artery and stenosis of the first diagonal branch which immediately was treated by percutaneous coronary intervention (PCI) using two stent bifurcation strategy (T and protrusion -TAP). Significant stenosis of heavy calcified right coronary artery (RCA), and chronic total occlusion (CTO) of the posterior left ventricular artery was also found. (Figure 1). The patient was admitted to coronary unit and a loading dose of prasugrel 60mg and acetyl salycine acid 300mg just after procedure was administered. Patient was hemodynamically stable but chest pain with significant intensity persisted during next 12 hours with ECG pointing to inferior left ventricle wall ischemia.
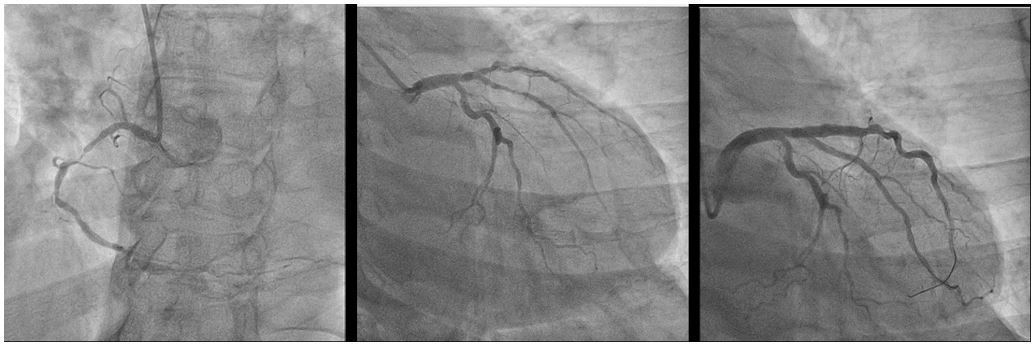
Figure 1: Initial coronarography. The left image shows significant stenosis of the right coronary artery. The middle image shows sub-occlusive stenosis of the left descending coronary artery and stenosis of the first diagonal branch. Right image shows successful reparation of both arteries using TAP stenting strategy.
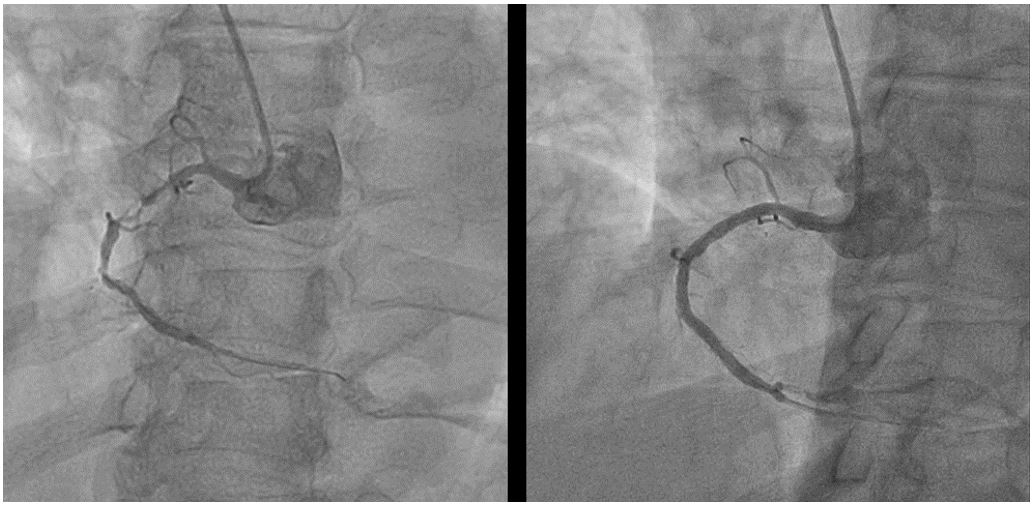
Figure 2: Percutaneous coronary intervention on right coronary artery. Left image shows significant stenosis of muddle part of right coronary artery. Right image shows successful reparation of right coronary artery after placing.
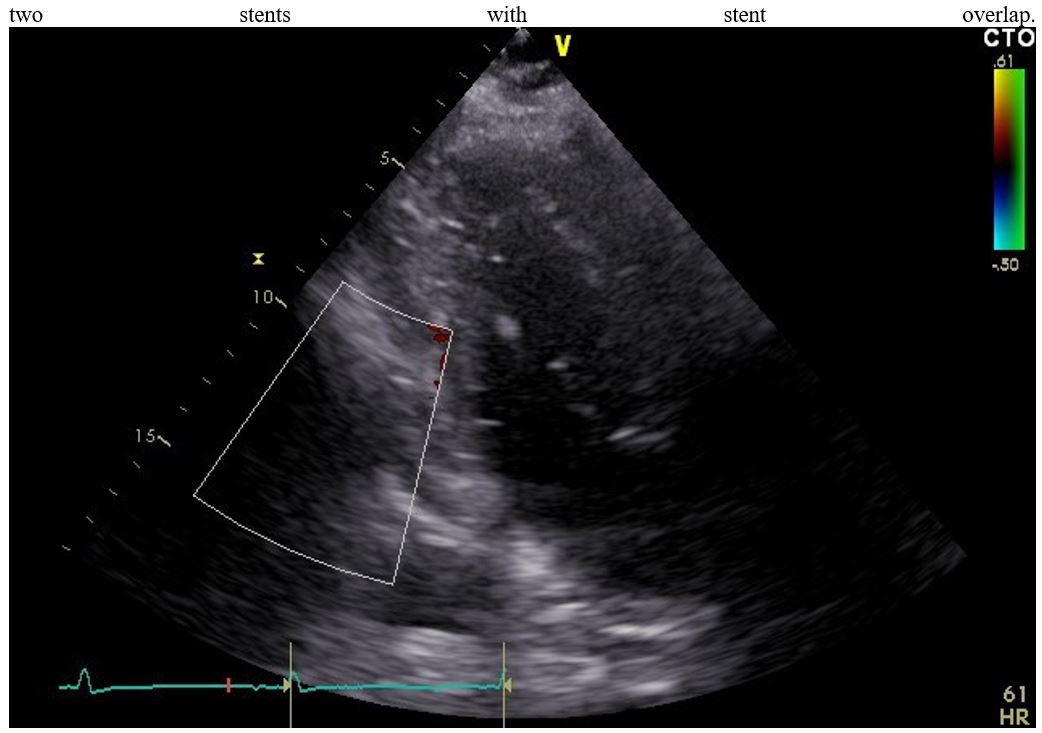
Figure 3: Bedside echocardiography showing sings of pericardial fluid.
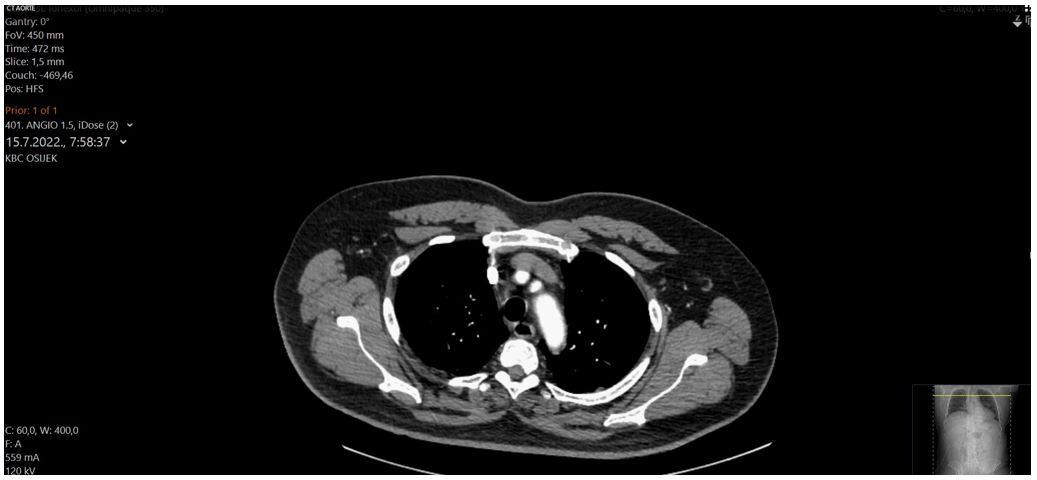
Figure 4: Images from aortic angiography showing no signs of rupture or dissection.
Next day after assessing renal function, new coronarography was made: left coronary tree was without stenosis with patent stents; we concluded that symptoms is causing significant stenosis the RCA. PCI was performed with the implantation of two stents and final TIMI III grade flow (Thrombolysis in Myocardial Infarction). After PCI patient reported feeling well with an improvement in symptoms (Figure 2.). However, four hours after the procedure, the patient experienced hypotension accompanied by echocardiographic sings cardiac tamponade with blood cloths in pericardial fluid (Figure 3.).
An urgent ultrasound-guided pericardiocentesis was made, with evacuation of 350 milliliters of blood following immediate hemodynamic stabilization of the patient. Repeated coronarography did not reveled signs of contrast extravasation pointing to arterial rupture, intravascular ultrasound (IVUS) or optical coherence tomography (OCT) were not available at the moment. CT angiography and ventriculography ruled out aortic or ventricular rupture or aortic dissection (Figure 4.). Next day tamponade recurrence occurred and 450 milliliters of bloody fluid was evacuated from the pericardium. The sample of fluid was subjected to microscopic analysis, revealing the presence of erythrocytes and granulocytes, while no malignant cells were detected. Considering the effusion and the patient's condition, a decision was made to deescalate dual antiplatelet therapy to clopidogrel only. The patient remained stable, and monitored in intensive coronary unit with daily pericardial effusion assessment. No tamponade recurrence was observed and on the seventh day pericardial drainage was removed with no pericardial fluid presence echocardiographic signs. Acetyl salycine acid in 100mg dose was carefully reintroduced. The patient was discharged four days later. The patient was closely monitored and followed up after one year of hospitalization with no recurrence of angina symptoms. Unfortunately, ten months later patient was diagnosed with colon cancer. The cancer surgery was successfully performed and he is under oncology treatment.
Discussion
Cardiac tamponade is a rare but serious complication of PCI. A recent Polish study reported that 0.11% of PCI was complicated by tamponade. Among these cases, 85% were successfully treated with pericardiocentesis, while the remaining 15% required surgical intervention [5]. An earlier United States study yielded similar results, with a 0.12% incidence of cardiac tamponade following PCI. Several risk factors for cardiac tamponade were identified: older age, multivessel disease, and complex coronary lesions [1, 6]. The aforementioned study revealed that in all patients who had tamponade in the catheterization laboratory the cause was apparent. However, among the group of patients who developed symptoms later (mean time of 4.4 hours after the PCI), for 10 of 14 patients had no apparent cause. Unfortunately, only seven of them underwent repeated coronarography to further investigate the underlying cause [6].
Our patient had unquestionable bleeding in the pericardium. Despite the absence of apparent perforation on repeated coronarography, it is possible that spontaneous closure occurred, as reported in a several cases [7, 8]. However, our patient experienced a recurrence of cardiac tamponade within a very short period of time, approximately 12 hours after the initial pericardiocentesis with an even greater amount of bloody fluid. Ruling out aortic or ventricular rupture or dissection, we cannot be sure about the actual cause of cardiac tamponade.
In some patients, tamponade occurs due to myocardial free wall rupture, which is most common complication of acute myocardial infarction or as a result of lesion caused by temporary pacemaker electrode in right ventricle, but that was not underlying causes in our case because no temporary pacemaker was used.
In conclusion, the exact cause of tamponade in our patient remains uncertain. However, considering the chronological events, it is possible that the PCI led to a small perforation in the coronary artery, which subsequently closed spontaneously after de-escalation of anti-aggregation therapy. Although we were unable to utilize intravascular ultrasound or optical coherence tomography in this case, had they been employed during the procedure, they could have potentially provided valuable insights into the underlying cause of tamponade.
References
- Adler Y, Charron P, Imazio M, Badano L, Baro´n-Esquivias G, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases. European Heart Journal. 2015; 36: 2921-2964.
- Orbach A, Schliamser JE, Flugelman MY, Zafrir B. Contemporary evaluation of the causes of cardiac tamponade: Acute and long-term outcomes. Cardiol J. 2016; 23(1): 57-63.
- Imazio M, Maria De Ferrari G. Cardiac tamponade: an educational review. European Heart Journal. Acute Cardiovascular Care. 2021; 10(1): 102-109.
- Ristic AD, Imazio M, Adler Y, Anastasakis A, Badano LP, et al. Triage strategy for urgent management of cardiac tamponade: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2014; 35: 2279-2284.
- Adamczyk M, Niedziela JT, Wasilewski J, Zembala M, Kalarus Z, et al. Prevalence, management and outcomes of cardiac tamponade complicating 66,812 invasive cardiac procedures: single-center clinical registry. Postepy Kardiol Interwencyjnej. 2021; 17(2): 193-199.
- Fejka M, Dixon SR, Safian RD, O’Neill WW, Grines CL, et al. Diagnosis, Management, and Clinical Outcome of Cardiac Tamponade Complicating Percutaneous Coronary Intervention. The American Journal of Cardiology. 2002; 90(11): 1183-6.
- Vivek Singh Guleriacorresponding and Prafull Sharma. Spontaneous closure of grade IV coronary perforation. Indian J Thorac Cardiovasc Surg. 2021; 37(1): 112-113.
- Li J, Zhang M, Kang X, Chen R, Wang F, Chen W. Pericardial tamponade after chronic total occlusion revascularization: a case report and literature review. Ann Palliat Med. 2021; 10(7): 8506-8511.

