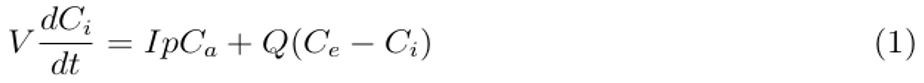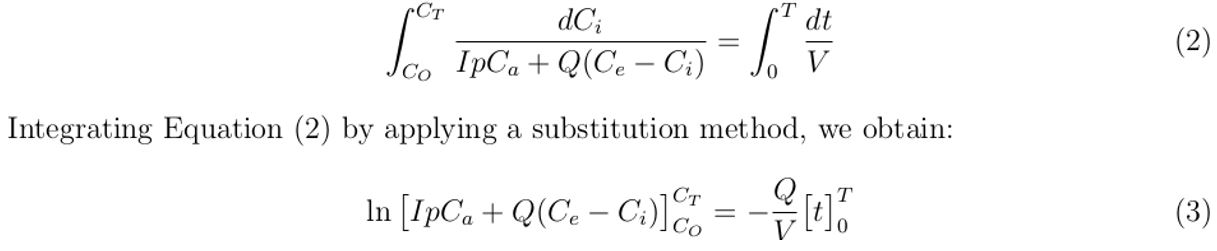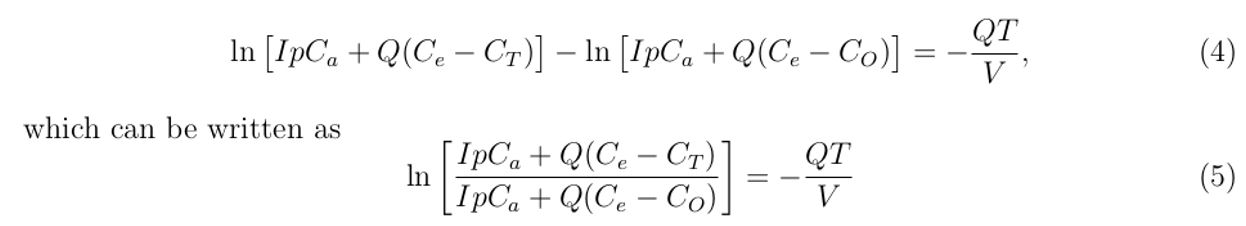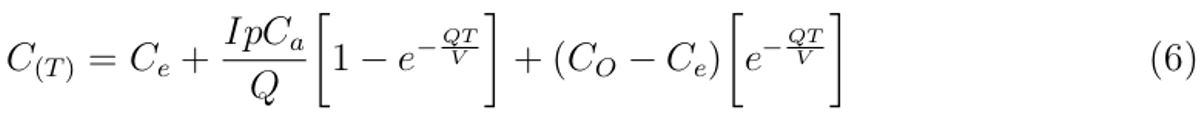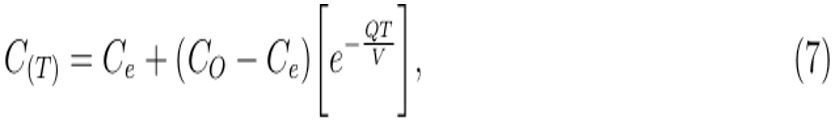Review Article - Volume 3 - Issue 6
Modelling the transmission and control of airborne infectious disease in healthcare settings
Chacha M. Issarow*
Department of Integrative Biomedical Sciences, Faculty of Health Sciences, University of Cape Town, South Africa.
Received Date : Oct 20, 2023
Accepted Date : Nov 27, 2023
Published Date: Dec 04, 2023
Copyright: © Chacha M. Issarow 2023
*Corresponding Author : : Chacha M. Issarow, Department of Integrative Biomedical Sciences, Faculty of Heath Sciences, University of Cape Town, South Africa.
Email: cissarow@gmail.com
DOI: Doi.org/10.55920/2771-019X/1592
Abstract
Backgraound: The transmission of airborne infectious diseases, such as tuberculosis (TB) and coronavirus disease 2019 (COVID-19), among healthcare workers (HCW) in healthcare settings (HCS), remains a public health concern. TB and COVID-19 transmission mostly occur in congregated and confined areas with limited ventilation per person through respiratory droplets from infectious individuals.
Methods: This study aims to use a mathematical modelling approach and carbon dioxide as a surrogate for exhaled air to investigate factors associated with the transmission of airborne infectious diseases, such as TB and COVID-19, in HCS and highlight preventive measures for disease control.
Results: This study found that high levels of interaction and duration of exposure to infectious individuals are among the factors accelerating airborne infectious disease transmission among HCW in HCS. Furthermore, it shows that an increasing number of infectious individuals, overcrowding, and insufficient ventilation are additional key factors contributing to the increasing airborne infectious disease incidence in HCS.
Conclusion: Adequate ventilation, a short duration of exposure, and successful treatment of identified cases are some of the most effective interventions for disease control in HCS and the community.
Keywords: Mathematical modelling; airborne infectious disease; Tuberculosis; COVID-19.
Introduction
Airborne infectious diseases, such as tuberculosis (TB) and coronavirus disease 2019 (COVID-19), mostly transmit in congregated and confined spaces with limited ventilation per person and a high accumulation of exhaled air that may contain airborne infectious particles [1,2]. TB and COVID-19 transmission occur through respiratory droplets from infectious individuals, and are highly correlated with social interactions, airspace per person, pathogen strains, proximity, frequency, and duration of exposure to infectious individuals [1, 3, 7, 8]. Healthcare settings (HCS) are one of the congregated and confined locations with limited ventilation per person where healthcare workers (HCW) interact and spend most of the time with patients. In these environmental conditions with a high concentration of exhaled air, some of which may contain infectious particles, airborne infectious disease transmission is assumed to be remarkably high given the presence of infectious and susceptible individuals.
The environment and modes of TB transmission are similar to COVID-19 transmission; the main difference is that COVID-19 is more contagious and can also be transmitted by touching contaminated surfaces and then touching your face afterwards. HCW, including nurses, doctors, and paramedics, are at high risk of being infected with and dying from infectious diseases due to frequent exposure and contact with patients in HCS3. For example, it was reported that many HCW became infected and many died during the COVID-19 outbreak in several countries as a result of exposure to infectious individuals in healthcare facilities [3].
This study aims to use carbon dioxide as a surrogate for exhaled air to model the transmission of airborne infectious diseases, such as TB and COVID-19, in HCS and highlight preventive measures for disease control. Carbon dioxide is one of the most diffusible tracer gases that is usually used to measure the quality of airspaces [1]. The assumption in this study is that all occupants in HCS, both infectious and non-infectious individuals, contribute equally to exhaled air. When the number of infectious cases increases, the concentration of exhaled air that may contain airborne infectious particles increases, and susceptible individuals (HCW and other susceptible individuals) may be infected if they inhale these infectious particles and reach a threshold level at the site of infection1. The analysis study differs from prior airborne infectious disease models, such as COVID-19 models, because it uses an approach that accurately depicts the transmission of airborne infectious diseases in real life.
Material and Methods
Modelling airborne infectious disease in exhaled air
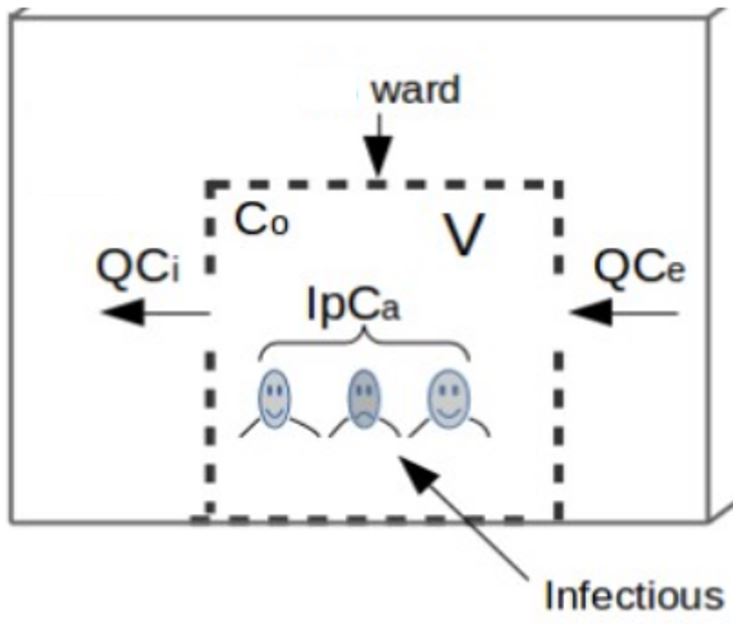
Raised carbon dioxide as a surrogate for exhaled air without the presence of an infectious source is a measure of air exchange between non-infectious individuals. The proportion of this air exchange, that is potentially infectious is determined by the number of infectious individuals and the prevalence of active cases in the healthcare building. This implies that disease transmission is directly correlated with the number of patients, such that the number of new cases increases dramatically with increasing prevalence, which is why TB transmission is remarkably high in high TB settings. Exhaled air contains approximately 40,000 ppm of carbon dioxide, compared to nearly 400 ppm in ambient air [1]. Let us consider a healthcare building with infectious and non-infectious individuals as illustrated in Figure 1 and use carbon dioxide as a surrogate for exhaled air to demonstrate disease transmission and control in HCS by monitoring exhaled air by infectious individuals in the building as follows:
Figure 1: Airborne infectious disease transmission in HCS, a schematic of a ward.
Mathematically, accumulated carbon dioxide as a surrogate for exhaled air in the healthcare building of volume V per given time is equal to the rate of exhaled air generated by occupants (infectious and susceptible individuals) that may contain airborne infectious particles, IpCa, plus ambient air in the building, QCe , minus indoor air removed by ventilation, QCi , as demonstrated in differential equation [1].
where Ci denotes indoor carbon dioxide concentration (ppm), Q is the ventilation rate (L/s), Ce is the outdoor carbon dioxide concentration (ppm), p is the breathing rate of individuals in the healthcare building (L/s), Ca is the carbon dioxide fraction contained in breathed air and I is a number of individuals (infectious and susceptible) in the healthcare area.
Arranging Equation (1) and integrating with respect to time (t) from t = 0 when CO2 = CO to t = T when CO2 = CT , we obtain the concentration of sampled exhaled air in the healthcare building as follows:
Simplifying Equation (3) further, gives:
Applying logarithmic and exponential rules in Equation (5) and simplifying it further by making CT the subject, we obtain an expression that describes the sampled exhaled air concentration in the healthcare building as:
where CO is the indoor carbon dioxide concentration at time, t = 0, which is greater than outdoor carbon dioxide concentration (Ce ).
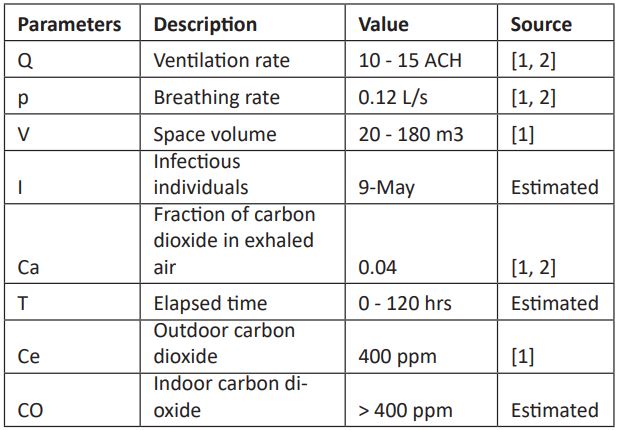
The quantity of sampled carbon dioxide as a surrogate for exhaled air in the space with high probability of causing airborne infectious disease in the presence of infectious individual can be obtained by substituting the values of the parameters described in Table 1 into Equation (6).
However, if the number of patients in the healthcare building can be treated with effective chemotherapy and cured then IpCa → 0 and Equation (6) becomes:
which describes the decay exponential curve as demonstrated in simulation results in Figure 3, showing that exhaled air that may contain airborne infectious particles decreases dramatically with decreasing number of infectious individuals in the healthcare building.
Table 1: Description of parameters and values used in the model development and simulation. Values of parameters were estimated from published literature and model fitting.
Results
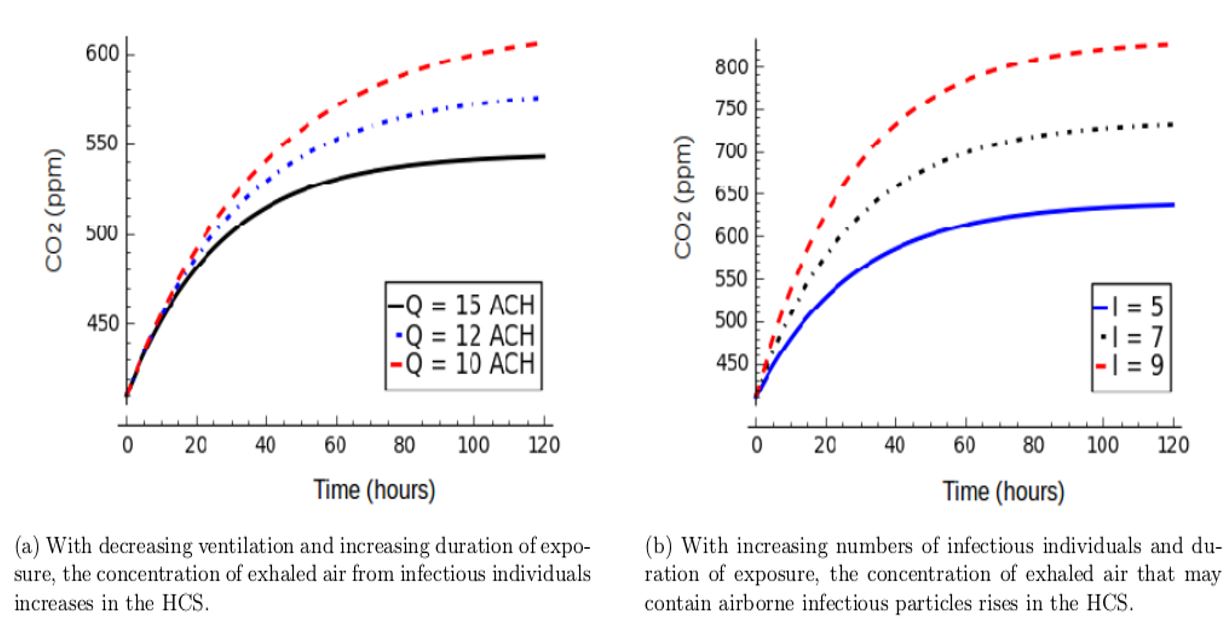
This study modelled the transmission of airborne infectious diseases, like TB and COVID-19, in HCS using carbon dioxide as a stand-in for exhaled air. This strategy differs from other airborne infectious disease models in that it takes into account the spread of airborne infectious diseases in real life. In model simulation (Equation 6), it shows that the concentration of exhaled air that may contain airborne infectious particles increases in the healthcare building as air change per hour (ACH) decreases with increasing duration of exposure (Figure 2a). In this environment, the risk of infection for exposed and susceptible individuals increases when the number and concentration of inhaled infectious particles reach a threshold level [1].
This study shows that when the number of patients increases over time in the healthcare building, the concentration of exhaled air that may contain airborne infectious particles increases, and a large number of exposed susceptible individuals may be infected (Figure 2b). This implies that ventilation should vary according to the number of occupants and should not be uniform in all HCS, as postulated by the WHO at 12 ACH.
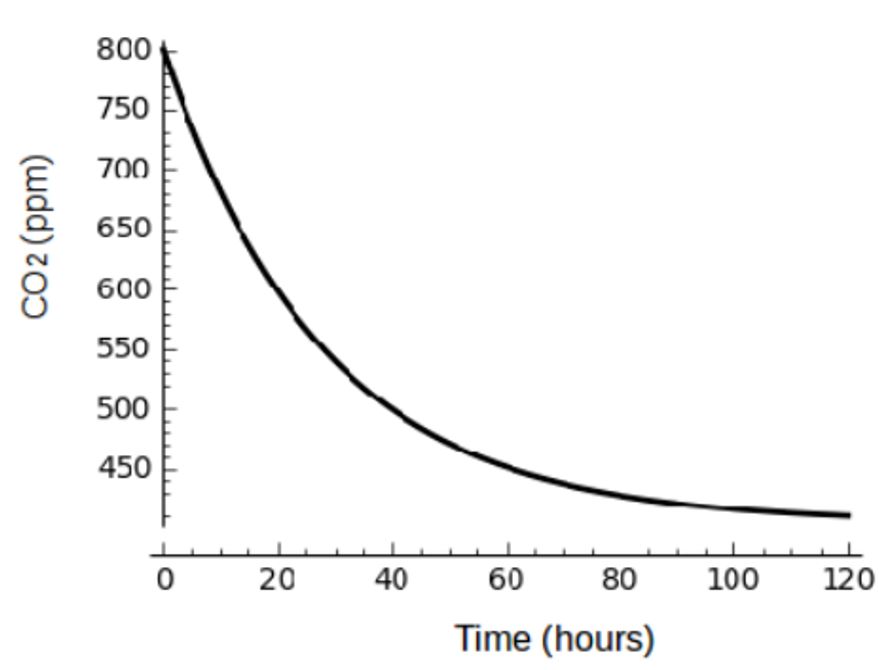
Simulation of Equation (7) in Figure 3 shows that if the number of patients can be treated, cured or reduced in HCS, the concentration of exhaled air decreases exponentially, suggesting that disease control can be achieved in these settings.
The transmission of airborne infectious diseases in HCS is highly correlated with high concentrations of air pollution, poor air disinfection, and a high number of infected individuals in the setting. The concentration of airborne infectious particles per volume of air increases dramatically with the increasing number of patients in a given airspace (Figure 2b). Unidentified cases and asymptomatic patients in HCS are additional factors that drive airborne infectious disease transmission among HCW. In these scenarios, the probability of HCW acquiring an airborne infectious disease becomes higher because preventive measures are not taken before exposure to infectious individuals. However, even if these cases are identified, some healthcare facilities cannot afford personal protective equipment (PPE), so HCW provide services and interact with patients in spaces with limited ventilation without wearing PPE. Furthermore, since the concentration of airborne infectious particles increases dramatically in the given space with increasing duration of exposure, HCW may become infected depending on the proximity of staff offices to patient wards, exposure frequency, interaction with patients, and virulence of strains of airborne infectious particles.
Although the WHO specified a ventilation rate of 12 ACH for HCS, most are constructed below standard and are insufficient for indoor air circulation and the removal of airborne infectious particles. However, though the WHO-recommended ventilation is achieved in some HCS, the prevalence of airborne infectious disease is still high. The major problem is that the WHO specified the ventilation but not the number of patients per ward, location, or room size. Because ward room size, number of patients per ward, location, and climatic conditions differ across the globe, this ventilation is likely insufficient for disease control. As a result, ventilation cannot be the same in all health-care facilities; rather, it should be tailored to the location, climatic conditions, room size, and estimated number of patients to be accommodated.
Figure 2: Factors attributable to the increase in carbon dioxide concentration as a surrogate for exhaled air that may contain airborne infectious particles, which cause infection and disease in HCS (Simulation of Equation (6)).
Figure 3: Carbon dioxide, as a surrogate for infectious individuals’ exhaled air, decreases in the HCS as the number of patients per given period of time decreases (Simulation of Equation (7)).
Discussion
This study explored factors associated with the transmission of airborne infectious disease in HCS using a mathematical modelling approach and carbon dioxide as a surrogate for exhaled air. Although it is not the most effective intervention for airborne infectious disease control, natural or mechanical ventilation plays a key role in disease control by reducing (diluting) the concentration of airborne infectious particles expelled by infectors. Mechanical air disinfections, such as negative pressure ionization and ultraviolet germicidal irradiation in healthcare wards, can act as disease control interventions and may replace natural ventilation if installed properly [4, 6]. The WHO recommend a ventilation rate of 12 ACH for HCS, including isolation wards, though in exceptionally hazardous areas, such as autopsy rooms, airflow rates should be increased to 15 - 25 ACH [4].
The transmission of airborne infectious diseases, such as TB, has been noted in different healthcare sections, including autopsy rooms and hospital wards [4, 12]. Several studies have demonstrated the spread of TB infection and disease among HCW, including nurses and medical students. For example, a study conducted in 1924 in Oslo, Norway, by Heimbeck, noted that from 220 tuberculin-negative tests from 420 nursing students on entry into the degree, 210 (95%) became tuberculin-positive by graduation [5, 12]. Furthermore, in his 1946 work, Heimbeck demonstrated that 105 (37%) of 284 nurses who were initially tuberculin-negative became infected and developed TB disease [12]. Based on these findings, he concluded that nurses are at excessively high risk of acquiring TB infection and disease. Apart from Heimbeck’s work, numerous other studies have reported higher rates of TB transmission risk among nurses than the general population, and one of the studies concluded that the risk of developing TB among nurses was 500 times higher than the general population. Another study in 1953 noted that the risk of developing TB for hospital workers in contact with patients, including nurses and technical workers such as doctors, is 8 to 10 times higher than for other workers in the same institution without such contact [12]. Numerous studies since then, including empirical evidence demonstrated by Myers et al [5] also show that HCW, such as nurses and doctors, are at a high risk of acquiring TB infection and developing clinical disease in their working environments. The main reservoir and spread of TB strains among HCW is not clearly understood; however, there are several factors and conditions attributable to TB transmission in HCS. Poor infrastructure is a key factor fostering airborne infectious disease transmission in HCS, particularly in low- and middle-income countries, where healthcare facilities are of poor quality and can accelerate the transmission of infectious diseases.
It is important to note that HCW can be infected from three main sources: the general population to HCW, patients to HCW, and from HCW to HCW. It is assumed that the highest transmission might be HCW to HCW in the tea rooms, which do not have high ventilation and require the removal of PPE, such as masks. Apart from HCW, susceptible individuals, such as HIV infected individuals, children, and the elderly, are at high risk of acquiring airborne infectious disease in the HCS because of sharing environments and thus expired air with patients. For example, due to either overpopulation or limited ward rooms in some HCS, particularly in developing countries, HIV-infected and TB patients are admitted to the same ward and share exhaled air, some of which is derived from infective sources. This is one of the factors fostering the spread of co-infection in HCS and the community at large. Furthermore, since they don’t receive treatment, unidentified and asymptomatic patients are an additional threat in HCS and in the general population [9]. Since they are not yet identified as cases for treatment and interact with people in different locations, this group may be among the unidentified super-spreaders in the community11.
Adequate ventilation, a short duration of exposure, identification, and successful treatment of cases are some of the most effective interventions for disease control in HCS and the community. If identified cases can be isolated and treated with effective chemotherapy, it will have a significant impact on disease control strategies. Separating infectious individuals from vulnerable and susceptible people may help reduce the rising number of new cases. Individuals infected with COVID-19, for example, should be separated from other patients, such as TB cases, because some of these people may have underlined conditions, increasing their risk of infection and developing active disease. The force of infection that accelerates airborne infectious disease transmission in HCS is driven by high disease prevalence and the virulence of infecting pathogen strains, and if cases can be successfully treated, this will potentially reduce the likelihood of new cases.
Competing interests: The authors declare no competing interests.
Acknowledgements: This (publication) was made possible (in part) by a grant from Carnegie Corporation of New York. The statements made and views expressed are solely the responsibility of the author.
References
- Sepkowitz KA, Tuberculosis and the healthcare worker: a historical perspective, Annals of internal medicine. 1994; 120(1): 71-79.
- Gammaitoni L, Nucci MC. Using a mathematical model to evaluate the efficacy of TB control measures. Emerg Infect Dis. 1997; 3: 335-342.
- Myers JA, The prevention of tuberculosis among nurses, The American Journal of Nursing. 1930; 1361-1372.
- Escombe AR, Moore DA, Gilman RH, Navincopa M, Ticona E, Mitchell B, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission, PLoS medicine. 2009; 6(3): e100004.
- Loudon RG, Roberts RM. Droplet explusion from the respiratory tract. The American review of respiratory disease. 1967; 95(3); 435-442.
- Hodgson MJ, Miller SL, Li Y, McCoy WF, Parsons SA, Schoen LJ, et al. Position document on airborne infectious disease. 2009.
- Dharmadhikari AS, Mphahlele M, Stoltz A, Venter K, Mathebula R, Masotla T, et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward, American journal of respiratory and critical care medicine. 2012; 185(10): 1104-1109, 2012.
- Vesley DL. Respiratory protection devices, American journal of infection control. 1995; 23(2): 165-168.
- Iseman MD. Invited commentary on Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward, American journal of epidemiology. 19895; 142(1): 1-2.
- Issarow CM, Mulder N, Wood R. Modelling the risk of airborne infectious disease using exhaled air. Journal of theoretical biology. 2015; 372: 100-106.
- Wells WF. Airborne Contagion and Air Hygiene. An Ecological Study of Droplet Infections. Airborne Contagion and Air Hygiene. An Ecological Study of Droplet Infections. 1955.
- Issarow CM, Transmission Dynamics and Control of COVID-19 Pandemic: A Mathematical Modelling Study. 10(3). AJBSR.MS.ID.001508. DOI:10.34297/AJBSR.2020.10.001508, 2020.