Case Report - Volume 3 - Issue 6
Autoimmune hepatitis: An atypical case report with diagnostic challenges
Maxime Doublier; Cléa Dubrou*; Daniel Bertin; Nathalie Bardin
Biogénopôle, Immunology Laboratory, Timone Hospital, Assistance Publique des Hopitaux de Marseille, Marseille, France.
Received Date : Nov 07, 2023
Accepted Date : Nov 30, 2023
Published Date: Dec 06, 2023
Copyright: © Cléa Dubrou 2023
*Corresponding Author : Cléa Dubrou, Biogénopôle, Immunology Laboratory, Timone Hospital, Assistance Publique des Hopitaux de Marseille, Marseille, France.
Email: clea-marie.dubrou@ap-hm.fr
DOI: Doi.org/10.55920/2771-019X/1594
Abstract
Autoimmune hepatitis (AIH) is a rare chronic liver disease characterized by liver inflammation resulting from an abnormal immune response of the affected patients against their own liver cells. Due to its rarity and the presence of nonpathognomonic clinical signs, the diagnosis remains a major challenge often resulting in delays in its identification and in the implementation of appropriate treatment. We report here a clinical case underlying the complexity of AHI diagnosis. The patient, a 45-years-old male, was admitted in gastroenterology department with symptoms including asthenia, splenomegaly and conjonctival subicteria, commonly associated with liver disorders, but also attributed to various other diseases. All this makes the diagnosis of autoimmune hepatitis difficult, and given the immunological findings, its characterization even more so.
Introduction
Autoimmune hepatitis (AIH) is a rare chronic liver disease characterized by liver inflammation resulting from an abnormal immune response of the affected patients against their own liver cells [1, 2]. This disease remains poorly understood, and its diagnosis is challenging due to the presence of nonspecific clinical signs and its low prevalence (about 20/100 000) [3]. Early and accurate identification of autoimmune hepatitis is essential for tailored therapeutic management to prevent disease progression and potential complications. The advancement of knowledge and the accumulation of successive clinical cases contribute to a better understanding of this rare disease, AIH, and thus to more effective therapeutic management [4]. In this case report, we present a clinical case of a 45-year-old man suspected of having AIH despite the absence of clear clinical signs. We highlight the diagnostic difficulties encountered during the patient's management due to the rarity of the disease and the non-specific nature of the observed clinical signs. This clinical observation highlight the importance of a thorough and methodical approach to achieve a precise diagnosis and provide appropriate treatment for patients with autoimmune hepatitis.
Case Presentation
A 45 year-old man with pancytopenia, elevated liver enzymes, alkaline phosphatase and gamma-glutamyl Transferase was referred to our university hospital. Clinically, the patient presented with asthenia, conjunctival subicterus and a 3 cm splenomegaly. His past medical history included hepatitis A (curedas a child), iron deficiency, Biermer's disease, and Hashimoto's thyroiditis. Regarding his family history, there is a pronounced autoimmune background since we note Biermer's disease in his mother and Basedow's disease in his brother. The patient has no clinical signs that might suggest autoimmune hepatitis, but this does not rule out the diagnosis. He has no drug or alcohol intoxication.Given the absence of other obvious causes of hepatitis (viruses, toxic substances, etc.), AIH is considered and further biological tests were performed. The patient is currently not receiving any specific treatment for their autoimmune disease. Instead, they are on supplementation thera
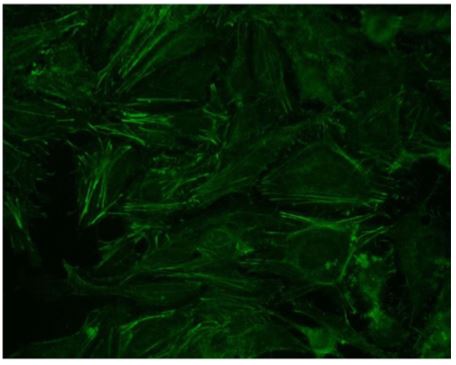
Figure 1: Indirect immunofluorescence on HEp-2 cells (x400). Cytoplasmic linear fibrillar pattern typical of anti-actin auto -antibody.
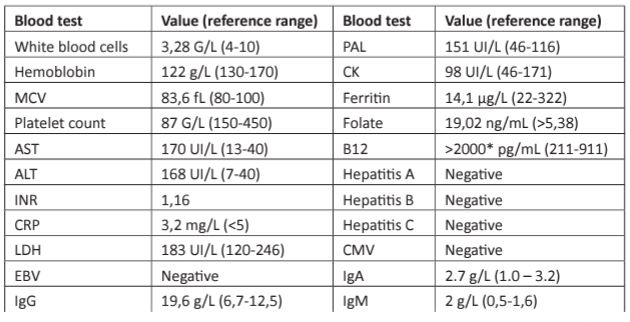
Table 1: Laboratory data.
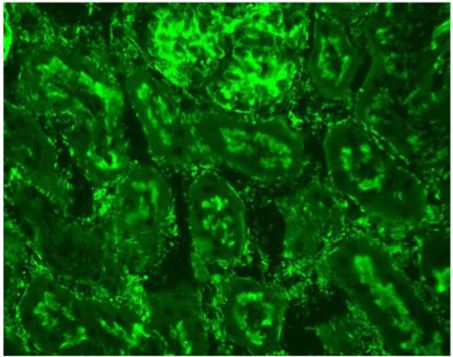
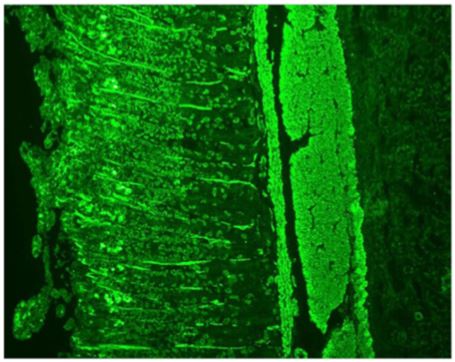
Figure 2: Indirect immunofluorescence on rat kidney tissue (x400). Peritubular fluorescent spicules typical of anti-actin auto-antibodies are visible.
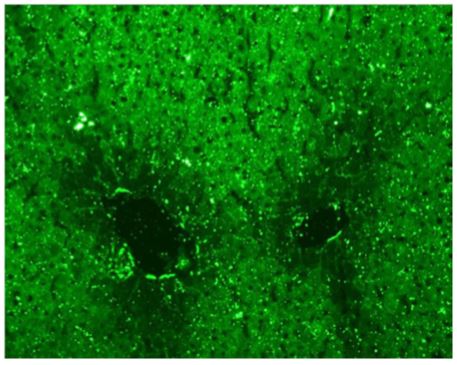
Figure 3: Indirect immunofluorescence on rat liver tissue (x400) showing a cytoplasmic staining of hepatocytes with a weakening of the stain around the centrilobular veins This pattern is typical of anti-LC1 autoantibodies.
Figure 4: Indirect immunofluorescence on rat stomach tissues (x100). Appearance consistent with anti-gastric parietal cell autoantibodies.
pies, which include levothyroxine, vitamin B12, and vitamin K. The antinuclear auto-antibodies were negative on HEp-2 cells but a positive cytoplasmic pattern compatible with anti-actin auto-antibodies was observed (Figure 1). Indirect immunofluorescence on rat LKS (liver-kidney-stomach) tissues was also performed and showed the presence of anti-actin autoantibodies at a titer of 1:400 (Figure 2) and anti-LC1 autoantibodies at a titer of 1:800 (Figure 3). These results were also confirmed by dot blot analysis. A staining of the stomach tissue of the rat LKS was also observed and revealed anti-gastric parietal cells autoantibodies compatible with Biermer’s disease previously diagnosed in this patient (Figure 4).
Discussion
The uniqueness of this case lies in the presence of two different autoantibodies, which usually correspond to two different types of AIH. In literature, AIH has been typically categorized into type 1 and type 2 based on the presence of anti actin and anti LC1 autoantibodies, respectively [5, 6]. Our patient's case thus adds to the complexity of AIH classification, suggesting possible overlap or a spectrum of the disease rather than distinct categories. The AIH classification does not change with the simplified autoimmune hepatitis score [7]. Such overlap or coexistence of autoantibodies in AIH has been infrequently reported. For instance, studies [8-11] have highlighted similar cases where patients presented with multiple autoantibodies or multiple autoimmune syndromes, challenging the conventional AIH categorization. Furthermore, these studies proposed that the presence of multiple autoantibodies could potentially indicate a more aggressive disease course, though the exact implications remain ambiguous. In comparison to our case, while our patient had a strong autoimmune background, his clinical presentation was relatively milder, emphasizing the variability in the disease presentation. Another notable aspect in our case was the patient's pronounced autoimmune background. Some studies suggested that individuals with an autoimmune predisposition could be at a heightened risk for AIH [12]. This reiterates the importance of a detailed personal and family medical history in patients suspected of AIH.
Conclusion
In conclusion, this case underscores the heterogeneity and complexity of autoimmune hepatitis. The presence of both anti actin and anti LC1 autoantibodies in our patient challenges the traditional classification of AIH into type 1 and type 2. It emphasizes the need for a more nuanced approach to AIH diagnosis, which considers the potential for overlap or a spectrum of the disease. Moreover, it underscores the importance of considering a patient's complete autoimmune background when suspecting AIH. Such cases highlight the evolving nature of our understanding of autoimmune diseases and call for continuous research and updating of diagnostic and therapeutic protocols. Disclosures Human subjects: All authors have confirmed that this study did not involve human participants or tissue. Conflicts of interest: No Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Floreani A, Restrepo-Jiménez P, Secchi MF, et al. Etiopathogenesis of autoimmune hepatitis. J Autoimmun. déc. 2018; 95: 13343. 10.1016/j.jaut.2018.10.020
- Czaja AJ. Diagnosis and Management of Autoimmune Hepatitis: Current Status and Future Directions. Gut Liver. mars. 2016; 10: 177-203. 10.5009/gnl15352
- Lv T, Li M, Zeng N, et al. Systematic review and meta-analysis on the incidence and prevalence of autoimmune hepatitis in Asian, European, and American population. J Gastroenterol Hepatol. 2019; 34: 1676-84. 10.1111/jgh.14746
- Sebode M, Hartl J, Vergani D, Lohse AW. International Autoimmune Hepatitis Group (IAIHG). Autoimmune hepatitis: From current knowledge and clinical practice to future research agenda. Liver Int Off J Int Assoc Study Liver. janv. 2018; 38: 15-22. 10.1111/liv.13458
- Dalekos GN, Zachou K, Liaskos C, Gatselis N. Autoantibodies and defined target autoantigens in autoimmune hepatitis: an overview. Eur J Intern Med. août. 2002; 13: 293-303. 10.1016/s0953- 6205(02)00089-4
- Ertem E, Ertem İS, Binicier ÖB. A rare case of autoimmune hepatitis presented with acute onset of high fever. Int J Infect Dis. 2014; 1: 152. 10.1016/j.ijid.2014.03.740
- Muratori P, Granito A, Pappas G, Muratori L. Validation of simplified diagnostic criteria for autoimmune hepatitis in Italian patients. Hepatol Baltim Md. mai 2009; 49(5): 1782-3. 10.1002/ hep.22825
- Sebode M, Weiler-Normann C, Liwinski T, Schramm C. Autoantibodies in Autoimmune Liver Disease-Clinical and Diagnostic Relevance. Front Immunol. 2018; 27: 2023.
- Manns MP, Vogel A. Autoimmune hepatitis, from mechanisms to therapy. Hepatol Baltim Md. févr. 2006; 43: 132-144. 10.1002/ hep.21059
- Oo YH, Hubscher SG, Adams DH. Autoimmune hepatitis: new paradigms in the pathogenesis, diagnosis, and management. Hepatol Int. 2010; 19: 475-93. 10.1007/s12072-010-9183-5
- Boberg KM, Chapman RW, Hirschfield GM, Lohse AW, Manns MP, Schrumpf E. Overlap syndromes: The International Autoimmune Hepatitis Group (IAIHG) position statement on a controversial issue. J Hepatol. 1: 374-85. 10.1016/j.jhep.2010.09.002
- Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. HLA, gut microbiome and hepatic autoimmunity. Front Immunol. 2022; 27: 2023.