Research article - Volume 4 - Issue 2
A Very Rare Case of Primary Mural Endocarditis of the Right Atrium due to Candida Albicans
Nikolaos Theodorakis M.D1*.; Georgia Vamvakou MD, Ph.D1; Arkadia Konstantopoulou M.D, Ph.D1; Eftychia Charatsi M.D2; Sophia Kalantzi M.D.2; Konstantinos Papadopoulos M.D, Ph.D3; Maria Nikolaou M.D,Ph.D1
1Department of Cardiology, Amalia Fleming General Hospital, 25is Martiou 14 str., 15127 Melissia, Greece.
2Department of Internal Medicine, Amalia Fleming General Hospital, 25is Martiou 14 str., 15127 Melissia, Greece.
3Department of Cardiology, Echocardiography Unit, Erythros Stavros General Hospital, Rousou 23 str., 11526 Athens, Greece.
Received Date : Dec 22, 2024
Accepted Date : Jan 08, 2024
Published Date: Jan 15, 2024
Copyright: © Theodorakis N 2024
*Corresponding Author : Nikolaos Theodorakis, Department of Cardiology, Amalia Fleming General Hospital, 25is Martiou 14 str., 15127 Melissia, Greece.
Email: nikolaostheodorakis1997@yahoo.com
DOI: Doi.org/10.55920/2771-019X/1608
Abstract
Right-sided infective endocarditis accounts for 5-10% of all infective endocarditis cases. Fungal endocarditis represents 1-3% of all of infective endocarditis cases, with Candida spp. being the most common microbiological cause. Primary mural endocarditis is defined by the absence of valvular vegetations together with the presence of vegetations on the endocardial free wall that are not associated with myocardial abscesses, cardiac structural abnormalities, devices, prosthetic material, thrombi or tumors. It constitutes a very rare entity and only 63 cases have been published the literature. C. albicans is the microbiological cause in only nine of these cases, none of which involves the right atrium. We report the case of a frail 84-year-old female patient with a pacemaker presenting with fever. In the transthoracic and three-dimensional transesophageal echocardiograms, a mobile mass was found attached on the free wall of the right atrial base. Blood cultures were positive for C. albicans and the modified Duke criteria were fulfilled for a definite diagnosis of infective endocarditis. After evaluation from a heart team the patient was managed with transvenous lead extraction and intravenous high-dose caspofungin, according to the 2023 Guidelines of the European Society of Cardiology for the management of endocarditis. Pacemaker lead cultures were negative leading to the diagnosis of primary mural endocarditis. The patient responded well to treatment with significant clinical and laboratory improvement. Long-term oral fluconazole was initiated after hospital discharge with a close follow-up plan. To our knowledge this is the first case of primary mural endocarditis of the right atrium due to C. albicans.
Abbreviations: CIED: Cardiac Implantable Electronic Devices; ESC: European Society of Cardiology; TTE: Transthoracic echocardiogram; TEE: Transesophageal echocardiogram
Introduction
Right-sided infective endocarditis represents 5-10% of all infective endocarditis cases, with the most common microbiological cause being S. aureus (75%) [1]. Fungal endocarditis accounts for 1-3% of all infective endocarditis cases, with Candida spp. being the most common microbiological cause (up to 50%) followed by Aspergillus spp. (25%) [2]. Endocarditis due to C. albicans is characterized by a high mortality (50%) [2,3]. Primary mural endocarditis is a very rare entity defined by the absence of valvular vegetations together with the presence of vegetations on the endocardial free wall that are not associated with myocardial abscesses, cardiac structural abnormalities (e.g., atrial or ventricular septal defects), cardiac implantable electronic devices (CIED), prosthetic material, thrombi or tumors [4]. Data from a 2017 review and a 2019 prospective cohort and review identified a total of 63 cases of primary mural endocarditis in the literature, highlighting the rarity of this entity. In the above studies, the average vegetation size was 21 mm and the most common cause was S. aureus (33%). C. albicans represented 14% of primary mural endocarditis cases (nine cases in total), while it accounts for only 2% of all infective endocarditis cases [2, 4, 5]. To our knowledge, this is the first case of primary mural endocarditis of the right atrium due to C. albicans, in a patient with a pacemaker who presented with fever.
Case presentation
An 84-year-old Caucasian female presented with a one-week history of fever. Her past medical included a dual-chamber pacemaker placed due to high-degree atrioventricular block, uncontrolled type 2 diabetes mellitus (HbA1c = 8.1%) under insulin therapy, arterial hypertension, hyperlipidemia, chronic kidney disease (stage IIIb), chronic obstructive pulmonary disease and hypothyroidism. The patient was characterized as frail based on the Fried Frailty Phenotype [6].
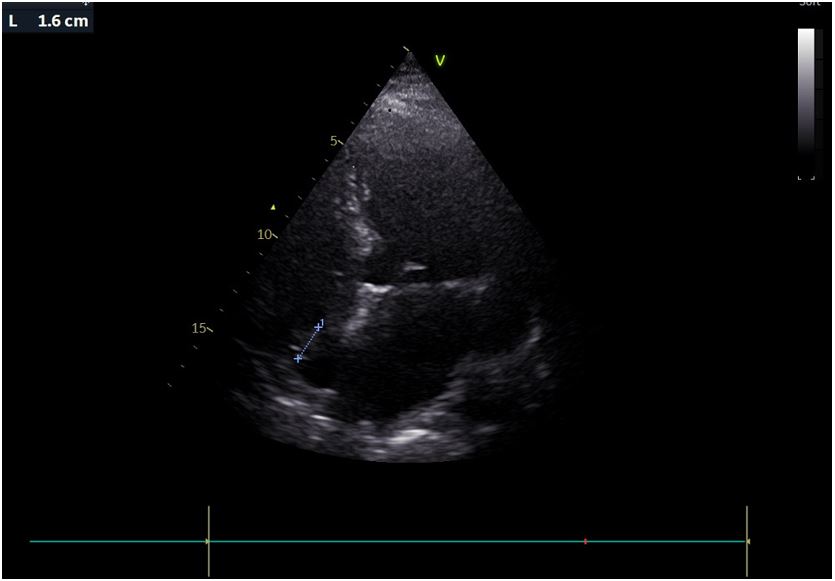
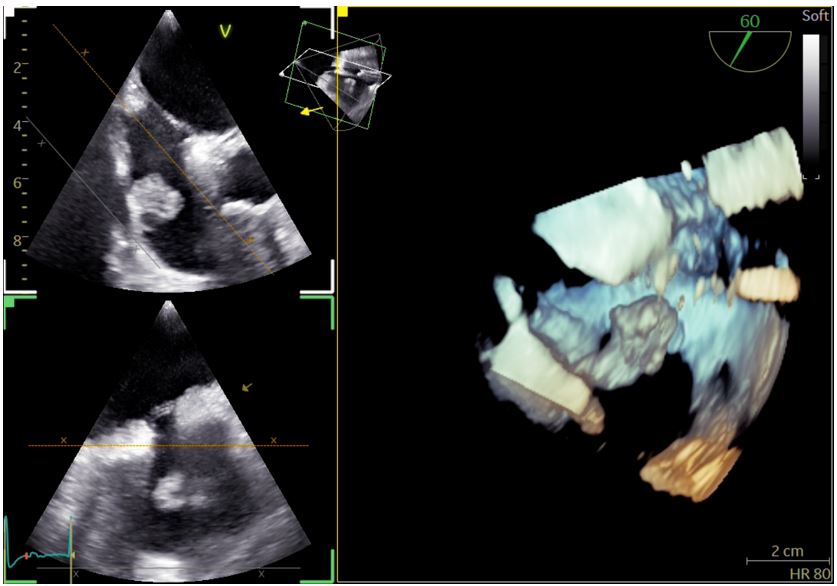
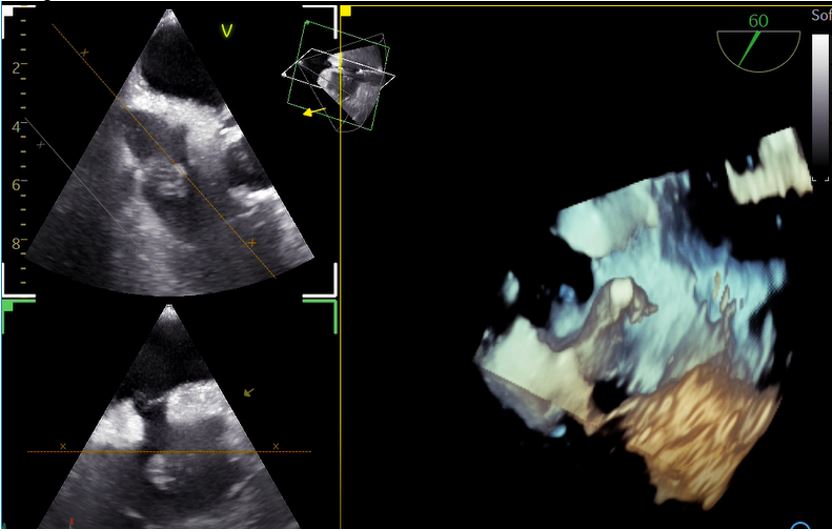
On admission she was hemodynamically stable and febrile (38.8oC), while clinical examination revealed extensive fungal fingernail infection, without the presence or murmurs, stigmata of infective endocarditis or signs of pocket infection. The initial investigations were significant for elevations of the erythrocyte sedimentation rate (66 mm/h) and C-reactive protein (52,9 mg/L, normal range <3,2 mg/L). The electrocardiogram showed normal sinus rhythm, urine cultures were negative, while a computed tomography scan of the chest, abdomen and pelvis did not reveal any significant abnormalities. Transthoracic echocardiography (TTE) was significant for the presence of a mobile mass on the base of the right atrium, measuring 16 mm on the largest dimension, as seen in Figure 1, which was not present on a TTE seven months prior to presentation. The transesophageal echocardiogram (TEE) confirmed the presence of the mass attached to the free wall of the right atrial base, without evidence of attachment to the eustachian or tricuspid valves. The above raised the clinical suspicion for infective endocarditis prompting initiation of empirical treatment with vancomycin and gentamicin, with close monitoring of serum levels, pending the blood culture results. At the same time, immediate removal of the pacemaker with transvenous lead extraction was performed, with the patient remaining electrically stable. A three-dimensional TEE was performed after pacemaker extraction, revealing patent foramen ovale and a bilobar mobile mass attached on the free wall of the right atrial base, near the inferior vena cava, without evidence of valvular involvement. The mass, measuring 18x20 mm, had a stem and spongy appearance at the top with the presence of vacuoles, as seen in Figures 2 and 3.
Blood cultures revealed the presence of for a multi-sensitive C. albicans strain. The sensitivity testing showed the following minimum inhibitory concentrations: ≤0.015 for anidulafungin, ≤0.008 for micafungin, 0.06 for caspofungin, 0.015 for voriconazole and 1 for fluconazole. The patient fulfilled the Modified Duke criteria for a definite diagnosis of endocarditis due to C. albicans: one major criterion (vegetation) and three minor criteria (presence of pacemaker, fever and positive blood cultures for C. albicans not fulfilling the major criteria) [7]. The cultures from the pacemaker leads were negative leading to the diagnosis of primary mural endocarditis of the right atrium due to C. albicans. According to the 2023 Guidelines of the European Society of Cardiology (ESC) for the management of endocarditis, intravenous caspofungin 100 mg once daily was initiated [7]. The patient responded well to treatment and became afebrile, with significant clinical and laboratory improvement, while all the following blood cultures were negative. After completing 4 weeks of intravenous caspofungin the patient was discharged with long-term oral fluconazole 200 mg once daily, based on the ESC Guidelines [7].
Figure 1: Transthoracic echocardiographic image of the vegetation on the base of the right atrial free wall.
Figure 2: Three-dimensional transesophageal echocardiographic image of the vegetation on the base of the right atrial free wall.
Figure 3: Three-dimensional transesophageal echocardiographic video of the vegetation on the base of the right atrial free wall.
Discussion
Due to its rarity, risk factors for endocarditis due to Candida spp. are not well established. One multivariable analysis suggests only valvular heart disease as an independent risk factor. Other possible risk factors include prosthetic heart valves, immunosuppression, diabetes mellitus, prolonged antibiotic therapy, intravenous drug use, previous cardiac surgery, vascular access lines and CIED [8, 9]. The most common Candida species causing endocarditis is C. albicans, followed by C. parapsilosis [3]. The diagnosis of endocarditis due to C. albicans was definite according to the modified Duke criteria. Diagnosis was further supported by the presence of risk factors (pacemaker, immunosuppression due to uncontrolled diabetes mellitus, chronic kidney disease and old age), extensive fungal fingernail infection, absence of other documented sites of active fungal disease explaining the candidemia, as well as significant clinical and laboratory improvement with antifungal treatment. Despite the presence of a pacemaker, our patient did not meet criteria for CIED-related fungal endocarditis, as the cultures from the explanted leads were negative. Primary mural endocarditis due to Candida spp. is very rare, with only nine cases identified in the literature [4, 5]. Primary mural endocarditis of the right atrium due to C. albicans has not been described in the literature and to our knowledge this is the first reported case.
Echocardiography is the main diagnostic tool for investigating right atrial masses [10]. Regarding our patient, the main diagnosis to differentiate was atrial myxoma, which is more common in the left atrium and usually originates from the interatrial septum with a narrow stalk [11, 12]. Atrial myxoma is a slowly growing tumor and the absence of a mass on a TTE seven months prior to the presentation further decreases the possibility of this diagnosis. Malignant cardiac tumors have an invasive nature and are usually associated with significant pericardial effusion and a more aggressive course which did not fit the patient’s description. Metastatic neoplasms are the most common malignant cardiac tumors with aggressive local behavior and often the presence of a primary tumor site, which was not the case for our patient. Angiosarcomas are the most frequent primary malignant cardiac tumors that usually arise from the right atrial free wall near the inferior vena cava but show infiltrative local behavior and often extend to the pericardium and right ventricle [11]. We should also highlight that the echogenicity of the mass with a spongy appearance and vacuoles was characteristic of a vegetation, compared to the compact echogenicity that characterizes many tumors. Another entity to exclude was intracardiac thrombus, which has different echogenicity and is more common in the left auricle, apex and akinetic segments of the left ventricular wall [11, 12].
Regarding the performance of biopsy for histological confirmation of the diagnosis, the patient was evaluated by a heart team taking into account multiple variables. The possibility of infective endocarditis was very high, considering the definite diagnosis from the modified Duke criteria together with the significant clinical and laboratory improvement with antifungal treatment. Another aspect that further supports the diagnosis is that Candida spp. is seven times more common in primary mural endocarditis (14% of cases), compared to the 2% frequency of all infective endocarditis cases [5]. On the other hand, the probability of an alternative diagnosis, such as a cardiac tumor or thrombus, was low, as already highlighted. Furthermore, the risk of performing biopsy was high, considering the elevated EUROSCORE of 33%, presence of severe comorbidities, frailty and patent foramen ovale. For the above reasons, following discussion with the patient and relatives, we decided against performing biopsy for diagnostic purposes.
The management of endocarditis due to Candida spp. is complex and warrants evaluation from a heart team. Based on the 2023 ESC Guidelines, liposomal amphotericin B with or without flucytosine or high-dose echinocandin is the management of choice, while long-term suppressive therapy with fluconazole or voriconazole is recommended, sometimes lifelong. Regarding CIED extraction, it is recommended with class I and grade B evidence in definite and class IIa and grade C evidence in probable CIED involvement [7]. According to expert consensus statements transvenous lead extraction can be performed in vegetations less than 2.5 cm, while larger vegetations require surgical extraction [13,14]. Our patient did not present with clinical or laboratory features of uncontrolled infection, acute heart failure, recurrent pulmonary emboli or other features to warrant cardiothoracic surgery for the purpose of treatment based on the 2023 ESC Guidelines [14]. We should highlight that specific guidelines on the management of primary mural endocarditis due to Candida spp. are lacking, due to the rarity of this entity.
Conclusion
Primary mural endocarditis due to Candida spp. is a very rare entity accompanied by high mortality rates. As a result, early diagnosis prompted by high clinical suspicion in the context of risk factors, as well as differential diagnosis from other cardiac masses is crucial for the prognosis. Management includes combination of antifungals and possible removal of any associated devices, while surgery is indicated in selected cases. We report the first case of primary mural endocarditis in the right atrium due to C. albicans, in a patient with a pacemaker who presented with fever, showing evidence of significant clinical and laboratory improvement with treatment.
Conflict of interest: None.
References
- Cimmino G, Bottino R, Formisano T, Orlandi M, Molinari D, et al. Current Views on Infective Endocarditis: Changing Epidemiology, Improving Diagnostic Tools and Centering the Patient for Up-to-Date Management. Life. 2023; 13(2): 377.
- Yuan SM. Fungal Endocarditis. Braz J Cardiovasc Surg. 2016; 31(3): 252-255.
- Halawa A, Henry PD, Sarubbi FA. Candida endocarditis associated with cardiac rhythm management devices: review with current treatment guidelines. Mycoses. 2011; 54(4): e168-74.
- Tahara M, Nagai T, Takase Y, Takiguchi S, Tanaka Y, et al. Primary Mural Endocarditis Without Valvular Involvement. J Ultrasound Med. 2017; 36(3): 659-664.
- Gutiérrez-Villanueva A, Muñoz P, Delgado-Montero A, Olmedo-Samperio M, de Alarcón A, et al. Mural Endocarditis: The GAMES Registry Series and Review of the Literature. Infect Dis Ther. 2021; 10(4): 2749-2764.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56(3): M146-56.
- Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, et al. 2023 ESC Guidelines for the management of endocarditis: Developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) Endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). European Heart Journal. 2023; 44(39): 3948-4042.
- Fuller R, Jacobs SE. Candida Infectious Endocarditis and Implantable Cardiac Device Infections. Mycopathologia. 2023; 188(6): 893-905.
- Rivera NT, Bray N, Wang H, Zelnick K, Osman A, et al. Rare infection of implantable cardioverter-defibrillator lead with Candida albicans: case report and literature review. Therapeutic Advances in Cardiovascular Disease. 2014; 8(5): 193-201.
- Shmueli H, Thomas F, Flint N, Setia G, Janjic A, et al. Right-Sided Infective Endocarditis 2020: Challenges and Updates in Diagnosis and Treatment. J Am Heart Assoc. 2020; 9(15): e017293.
- Pino PG, Moreo A, Lestuzzi C. Differential diagnosis of cardiac tumors: General consideration and echocardiographic approach. J Clin Ultrasound. 2022; 50(8): 1177-1193.
- Sykora D, Chaliki HP, Cummings KW, Sell-Dottin K, Stanton ML, et al. Incidentally Discovered Right Atrial Mass: A Rare and Unexpected Etiology. Tex Heart Inst J. 2023; 50(2): e217735
- Wilkoff BL, Love CJ, Byrd CL, Bongiorni MG, Carrillo RG, et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009; 6(7): 1085-104.
- Kusumoto FM, Schoenfeld MH, Wilkoff BL, Berul CI, Birgersdotter-Green UM, et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017; 14(12): 503-551.