Research article - Volume 4 - Issue 2
The off-label use with dabigatran in severe mitral stenosis
Wenjie Liu; Yunke Shi; Yunzhu Peng; Xuan Chen; Changyong Wu; Yimin Li; Qiongling Wang; Ruijie Li*; Huang Sun*
The First Department of Cardiology, The First Affiliated Hospital of Kunming Medical University, Kunming, China.
Received Date : Feb 09, 2024
Accepted Date : Feb 28, 2024
Published Date: Mar 06, 2024
Copyright: © Li R & Sun H 2024
*Corresponding Author : Ruijie Li The First Department of Cardiology, The First Affiliated Hospital of Kunming Medical University, Kunming, China.
Email: liruijie@ydyy.cn
DOI: Doi.org/10.55920/2771-019X/1642
Abstract
Anticoagulant therapy should be initiated in moderate to severe MS patients. The guideline recommends NOACs only for patients with non-valvular atrial fibrillation, only VKA preparations, such as warfarin, are recommended for valvular atrial fibrillation patients. This case study presents the anticoagulation therapy with dabigatran in severe mitral stenosis.
Learning Objectives
1.Dabigatran could be used as an alternative anticoagulant therapy in case of intolerance or suboptimal anticoagulation with VKA in those patients with severe mitral valve stenosis and/or with atrial fibrillation or left atrial thrombus.
2. Preoperative TEE or CTA evaluation is extremely important for mitral valve stenosis patients with PMBV
Case report
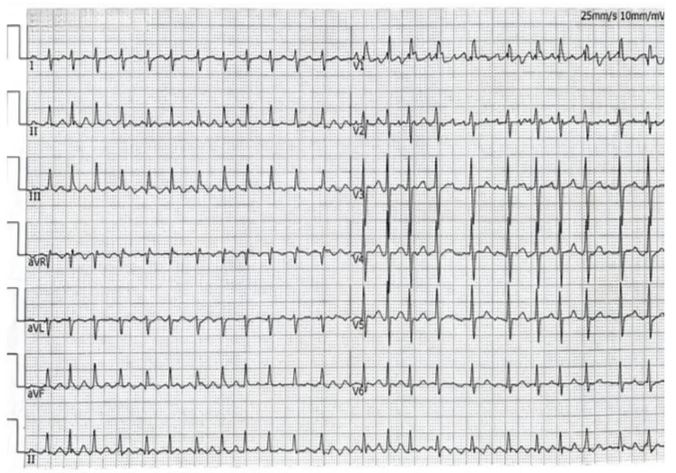
History of presentation: A 41-year-old Chinese woman presenting with untreated shortness of breath or dyspnea after activities for 5 years and with worsening palpitation symptoms accompanied by edema of both lower limbs for 2 weeks was admitted to our Cardiology department. The patient’s vital signs on presentation included blood pressure of 95/77mmhg, heart rhythm is irregularly irregular with a loud S1 and low-pitched diastolic murmur at the apex; Bilateral inspiratory crackles in the lower lung fields; Moderate edema of both lower limbs. The rest of the physical examination did not show any obvious abnormalities. The patient's electrocardiogram on admission was atrial fibrillation (Figure 1).
Past medical history
The patient used to be physically healthy and had no family history of genetically related diseases and no history of trauma and surgery.
Differential diagnosis
Conditions that can present with symptoms and signs similar to those with rheumatic MS should be included in the differential diagnosis: Severe mitral annular calcification, Congenital MS, Infective endocarditis, Left atrial myxoma and Cor triatriatum.
Investigations
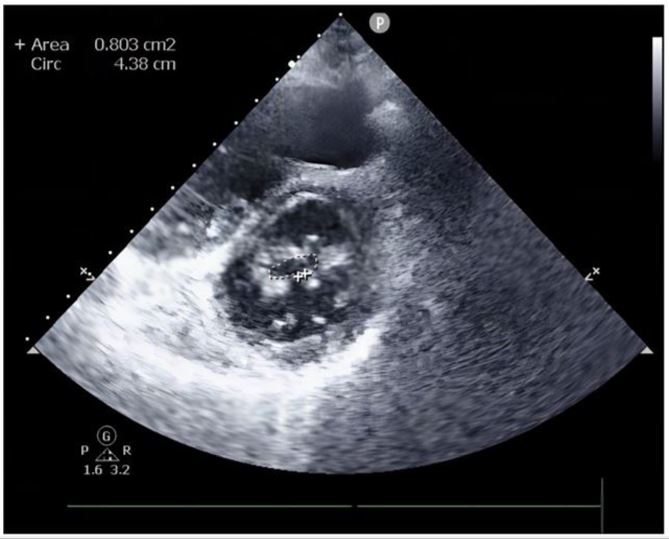
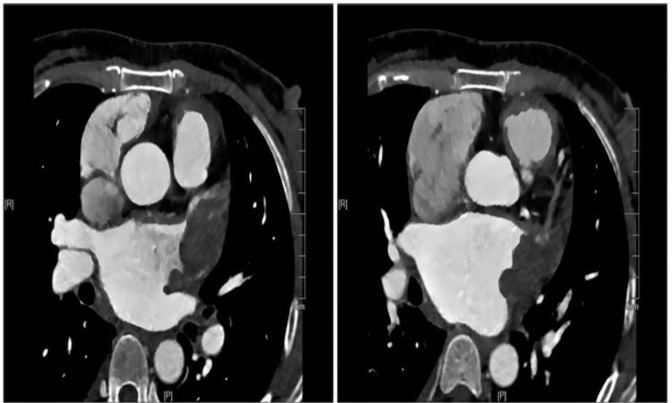
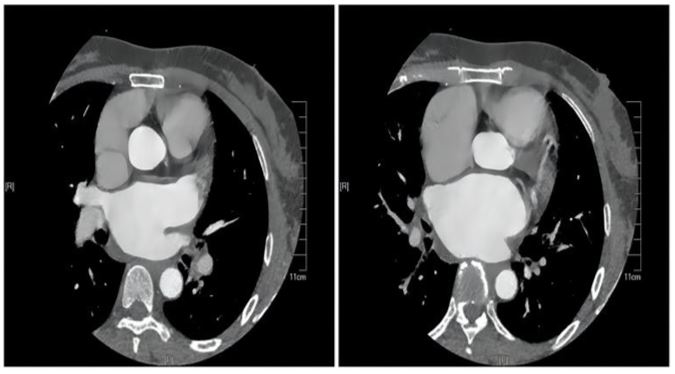
The initial laboratory data revealed the following: Brain natriuretic peptide(BNP, abnormal,<100pg/ml)of 882.34 pg/ ml, Alamine aminotransferase (ALT,abnormal,7.00-40.00 IU/L)of 141IU/L, Aspartate aminotransferase(AST, abnormal ,13.00-35.00IU/L )of 87IU/L, Creatinine(CREA,normal,71.00115.00umol/l) of 72umol/l, INR of 1.21. The Transthoracic echocardiography (TTE) showed:1. Rheumatic severe mitral stenosis with mild aortic regurgitation, mitral valve area of 0.8cm2 (The planimetry in parasternal short axis view)(Figure 2);2.The LVEF of 70% and the pulmonary artery pressure of 57mmhg.The patient didn’t undergo the transesophageal echocardiography(TEE) due to intolerance. The CTA of heart and pulmonary artery examination showed both atrial enlargement, thromboses found in the left atrial appendage and left atrium,
Figure 1: Electrocardiogram on admission.
Figure 2: TTE on admission.
Figure 3: CTA on admission.
with a maximum range of 42*25mm(Figure 3), and pulmonary embolism in the upper lobe and middle lobe of the right lung.
Management
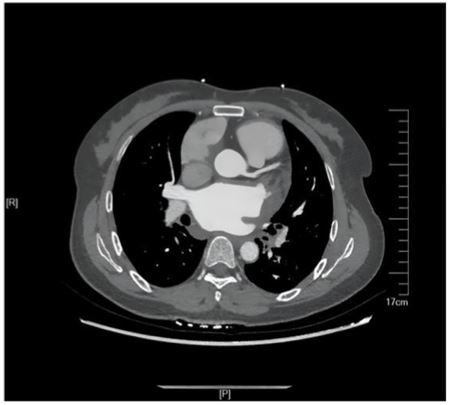
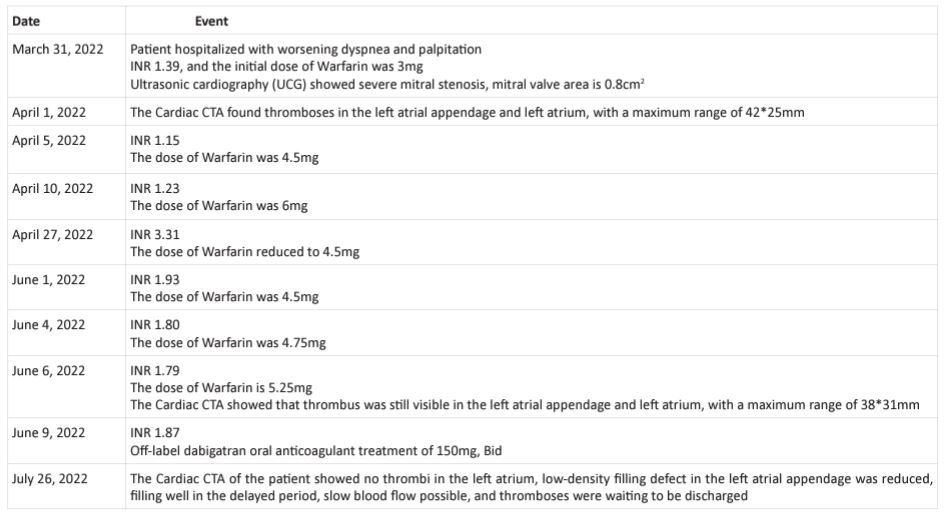
The patient had severe rheumatic mitral stenosis with heart failure, followed by left atrial and left atrial appendage thrombosis and pulmonary embolism. After admission, we administered furosemide 40mg, iv, QD diuretic treatment, and metoprolol 47.5mg, QD orally to reduce cardiac oxygen consumption and improve long-term prognosis. Initially, warfarin 3mg, QD, and oral anticoagulant treatment were given. According to recommendations 1 and 2 of the guidelines, for patients with severe mitral stenosis (MVA<1.5cm2) and symptoms, PMBV surgery can be performed if the valve shape and condition are appropriate and there is no left atrial thrombus. After communicating with the patient and their family members, the surgical hospital strongly emphasized that the patient had left atrial and left atrial appendage thrombosis and pulmonary embolism, and effective anticoagulation treatment became the primary goal of the patient. However, after taking warfarin orally, during the continuous follow-up and followup process from March 31, 2022 to June 9, 2022, the patient repeatedly adjusted the dosage of warfarin, and the patient's INR was still unable to maintain at 2-3. Furthermore, the followup CTA examination still showed the presence of thrombus in the left atrium and left atrial appendage (Figure 4, Table 1). Due to the patient's strong desire for early PMBV surgery as they are seeking medical attention from outside the city, on July 9, 2022, with the assistance of a joint consultation between the hematology and pharmacy departments, we reached a
Figure 4: CTA on June 6, 2022.
Figure 5: CTA on July 26, 2022.
consensus with the patient and their family to use the off-label use of dabigatran in patients with severe mitral stenosis, with a dose of 150mg and bid orally. During the use of dabigatran, the patient did not experience any bleeding events. After 45 days of medication therapy, a follow-up CTA examination on July 26, 2022 showed that the thrombus in the left atrial appendage and left atrium was basically absorbed(Figure 5), meeting the conditions for PMBV surgery. Subsequently, the patient underwent PMBV surgery under general anesthesia, and the mitral valve area recovered to 1.6 cm2 after surgery.
Discussion
We report a rare case of use dabigatran in severe mitral stenosis. The etiology of mitral stenosis usually is rheumatic heart disease. With the progress of the disease, most patients are involved in severe complications such as pulmonary edema and embolism. Persistent dyspnea decreased activity endurance, and other symptoms also seriously decline their quality of life. For the treatment of MS, medical therapy can improve the patient's symptoms but has little effect on the hemodynamic situation.
In the case we present above, the patient had thromboses in the left atrium and left atrial appendage. Thrombogenesis of mitral stenosis due to the mechanical obstruction which aggravates blood stasis that stimulates the coagulation system and also elevates the atrial pressure leading to endocardium damage. Secondly, in MS patients combine with atrial fibrillation, the atrial blood flow is slow and stasis which could
Table 1: Timeline.
activate the platelet function[1] that will develop thrombus form further. Anticoagulant therapy should be initiated in MS patients with left atrial or left atrial appendage thrombosis or atrial fibrillation without clear contraindications. The guideline explicitly recommends NOACs only for patients with nonvalvular atrial fibrillation, the term ‘non-valvular AF’ refers to AF in the absence of a mechanical prosthetic heart valve or moderate to severe mitral stenosis (usually of rheumatic origin) [2]. Only VKA preparations, such as warfarin, for valvular atrial f ibrillation patients, are recommended because phase III trials were currently excluded from mechanical prosthetic heart valve or moderate to severe mitral stenosis [3-6]. However, there is no RCT indicating that NOACs are less efficacious in patients with rheumatic mitral stenosis, and no rational base on which to hypothesize a differential response to NOACs vs. VKA [7]. Warfarin is a widely anticoagulant drug. The anticoagulant effect is mainly to competitively inhibit the functional activity of vitamin K-dependent coagulation factors (II, VII, IX, X), it can also inhibit proteins C and S. xtensive inhibition of coagulation factors increases the anticoagulant effect of warfarin, but also inevitably increases the risk of bleeding. And warfarin may increase the risk of thrombosis due to the inhibition of proteins C and S [8]. On the other hand, as a coumarin oral anticoagulant drug, warfarin interacts with a variety of drugs and foods, which not only affects the therapeutic effect but also easily causes adverse reactions. This may be the reason why the INR of the patient in this case repeatedly failed to reach 2-3. However, in the process of diagnosis and treatment, we should do the genetic test of warfarin to further clarify. Dabigatran is a specific, reversible, direct thrombin inhibitor that inhibits both free and fibrin-bound thrombin. According to Connolly SJ’s study [5], the benefit of dabigatran may be explained in part by the twice-daily dosing regimen. Since dabigatran has an elimination half-life of 12 to 17 hours, twice-daily dosing reduces variability in the anticoagulation effect, especially as compared with the anticoagulation effect of warfarin, which is difficult to control. By selectively inhibiting only thrombin, dabigatran may have antithrombotic efficacy while preserving some other hemostatic mechanisms in the coagulation system and thus potentially mitigating the risk of bleeding [5]. In our present case, After the use of dabigatran, thrombus absorption was confirmed by CTA review only 45 days, followed by no adverse bleeding events.
Follow-up
After PMBV, the hemodynamics and symptoms of the patient were significantly improved, and the patient was discharged from the hospital. Follow-up was not conducted because the patient returned to other provinces.
Conclusion
In our present case, dabigatran can even dissolve the LAA thrombi in patients with severe mitral stenosis, indicating that this drug could be a therapeutic option for patients with mitral stenosis. However, additional randomized data are needed to prove these preliminary findings before its use can be recommended in patients with rheumatic mitral stenosis.
References
- Sohara H, Amitani S, Kurose M, Miyahara K. Atrial fibrillation activates platelets and coagulation in a time-dependent manner: a study in patients with paroxysmal atrial fibrillation. J Am Coll Cardiol. 1997; 29(1): 106-12. Epub 1997/01/01. doi: 10.1016/ s0735-1097(96)00427-5. PubMed PMID: 8996302.
- Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace. 2021; 23(10): 1612-76. Epub 2021/04/26. doi: 10.1093/europace/euab065. PubMed PMID: 33895845.
- He Q, Sze CY, Shum TY, Hao G, Wong NB, Sin TH, et al. Comparing clinical outcomes of NOACs with warfarin on atrial fibrillation with Valvular heart diseases: a meta-analysis. BMC Cardiovasc Disord. 2019; 19(1): 113. Epub 2019/05/17. doi: 10.1186/s12872019-1089-0. PubMed PMID: 31092194; PubMed Central PMCID: PMC6521383.
- Ezekowitz MD, Nagarakanti R, Noack H, Brueckmann M, Litherland C, Jacobs M, et al. Comparison of Dabigatran and Warfarin in Patients With Atrial Fibrillation and Valvular Heart Disease: The RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulant Therapy). Circulation. 2016; 134(8): 589-98. Epub 2016/08/09. doi: 10.1161/CIRCULATIONAHA.115.020950. PubMed PMID: 27496855.
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361(12): 1139-51. Epub 2009/09/01. doi: 10.1056/NEJMoa0905561. PubMed PMID: 19717844.
- Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013; 369(13): 1206-14. Epub 2013/09/03. doi: 10.1056/NEJMoa1300615. PubMed PMID: 23991661.
- De Caterina R, John Camm A. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation accompanying mitral stenosis: the concept for a trial. Europace. 2016; 18(1): 6-11. Epub 2015/10/10. doi: 10.1093/europace/euv288. PubMed PMID: 26450845.
- Vidal A, Vanerio G. Dabigatran and left atrial appendage thrombus. J Thromb Thrombolysis. 2012; 34(4): 545-7. Epub 2012/05/31. doi: 10.1007/s11239-012-0747-1. PubMed PMID: 22644719.