Research article - Volume 4 - Issue 2
Musculoskeletal loading from road cycling and distance running on bone bending strength and BMD in athletes
Michael T.C. Liang*; Kevin Lewis; Alexandra Auslander; Jose Rocha-Rangel
Department of Kinesiology and Health Promotion, California State Polytechnic University, Pomona, CA, USA.
Received Date : Feb 01, 2024
Accepted Date : Mar 01, 2024
Published Date: Mar 08, 2024
Copyright: © Ghita SH 2024
*Corresponding Author : Michael TC Department of Kinesiology and Health Promotion, California State Polytechnic University, Pomona, CA, USA.
Email: mtcliang@cpp.edu
DOI: Doi.org/10.55920/2771-019X/1644
Abstract
Purpose: The purpose of this study was to determine whether chronic sport training comprising of low or nonimpact musculoskeletal loading in road cycling (CYC) and distance running (RUN) were associated with tibial and ulnar bending strength and calcaneus and wrist BMD in male athletes. Method. Study subjects included 12 road cyclists (CYC), 12 distance runners (RUN) and 12 physically active nonathletic (CON). Tibial and ulnar bending strength (EI, Nm2) were assessed using a mechanical response tissue analyzer (MRTA), and BMD (g/cm2) of the wrist and calcaneus were assessed using a peripheral X-ray absorptiometry. Group means differences among the study groups were determined using ANCOVA. Results. Ulnar bending strength (EI, Nm2) of CYC were higher than CON (62.0 ± 20 vs 44.7 ± 9 Nm2, p< 0.05), but not RUN. Tibial bending strength (EI, Nm2) of CYC and RUN were not higher than CON. Calcaneus BMD of RUN was higher than CON (0.666 ± 0.07 vs 0.579 ± 0.06 g/m2, p<0.05), but not CYC. Wrist BMD of CYC was not higher than (p = 0.07) RUN and CON. Conclusion. Chronic engagement in CYC training induces osteogenic adaptation for increased ulnar bending strength and RUN training induces increased calcaneus BMD.
Introduction
Osteoporosis and osteoporotic-related fractures are a considerable health concern worldwide [1] and cause increased morbidity, mortality and cost for society [2]. Epidemiological studies have suggested that achieving a high peak bone mass during growth with physically active lifestyle such as exercise training or sport participation might decrease the risk of developing osteoporosis and therefore osteoporotic fractures later in life [3 ,4]. Depending on the site of the skeleton, human acquired approximately 80-90 % of peak bone mass (PBM) during late adolescence [5]. Three major factors that contribute to PBM development including (1) genetics (2) nutrition, and (3) physical activity [6]. The last two factors are considered modifiable factors. It has been reported that sport participation or exercise training during adolescence and early adulthood can significantly enhance bone mineral content (BMC) and bone mineral density (BMD), both of which may be maintained into adulthood [5, 6]. Sport participation such as soccer, volleyball, tennis, running and swimming are considered among the most popular sports performed by adolescents in the USA [7, 8, 9]. However, participation in these sports may have different effects on bone development [8]. For example, participation in “osteogenic” sports, such as soccer, volleyball and running, can augment BMD and bone bending strength at the loaded sites of the skeleton [10, 11]. However, it is unknown if participation in “low-impact loading” sports, such as road running may have an impact on PBM and bone bending strength, and in “non-impact loading” sports, such as road cycling may compromise the achievement of PBM and bone bending strength. The application of a non-invasive mechanical response tissue analyzer (MRTA) quantifying the relationship between tibial and ulnar bone bending strength and wrist and calcaneus BMD has been reported in well-trained female athlete in gymnastics and synchronized swimming [12] and in well-trained female athletes in soccer, volleyball, and distance running [11]. In sedentary women, Miller et al. [13] employed the MRTA technology to assess ulnar bending strength after 20 weeks of isokinetic training of the dominant arm and reported an increase in the trained ulnar bending strength. However, Dutto et al. [14] employed the same technology to assess tibial bending strength after 26 weeks of high-intensity step aerobics training and reported no increase in tibial bending strength. The utility of MRTA technology has been reported elsewhere [15-17]. The MRTA technology has been cross-validated in monkey tibia with high correlation with the three-point bending stiffness test [15,16]. Locks et al. [16] compared the MRTA measurements of bending stiffness in human ulna bones to measurements made by ex vivo quasi-static mechanical testing (QMT) to quantify the reproducibility (i.e., precision and repeatability) of both methods, and reported results of the MRTA technology that have unmatched accuracy in QMT-validated measurements of bending stiffness with r2 = 0.999 of the human ulna bones. Because of its non-invasive technology, zero radiation exposure to the participants, and low instrumentation cost the MRTA was utilized for this study.
From a public health perspective, understanding how participation in sports training and competition among youth affect bone development is of great importance for developing high peak bone mass and bone health later in life. Crosssectional studies have evaluated differences in BMC between adolescents engaged in different sports in comparison to a non-sport participation control group [18,19]. There is a scarcity of report on the effect of regular engagement in sport training with low-impact or non-impact musculoskeletal loading sport initiated after the adolescent years such as longdistance running and road cycling in men on bone bending strength and BMD. Also, there are limited studies investigating the relationship between bone bending strength and BMD in the upper (nonweight-bearing) and lower (weight-bearing) extremities of male athletes regularly participating in lowimpact loading (i.e., distance running) and non-impact loading (i.e., road cycling) sports. According to Nikander et al [19] who classified distance running as a “repetitive single direction low-impact loading sport loading sport” and road cycling as a “repetitive” single direction non-impact loading sport”.
Purpose: The purpose of this study is to investigate the association of road cycling and long-distance running on ulnar and tibial bending strength and bone mineral density (BMD) of the wrist and calcaneus in young male athletes chronically trained and competed in road cycling and distance running and in young physically active male non-athletes. The study hypotheses are that 1) distance runners would have higher t ibial bending strength and calcaneus BMD than cyclists and non-athletes; 2) road cyclists would have higher ulnar bending strength and higher wrist BMD than distance runners and nonathletes; and 3) non-athletes would have the lowest values in ulnar and tibial bending strength, and lowest values in wrist and calcaneus BMD compared to the athletes.
Methods
Subjects: Twenty-four male athletes (age ranges from 18 – 30 years) volunteered to serve as study subjects. The athletes were United States National Collegiate Athletic Association (NCAA) Division II distance runners (RUN, n = 12), and Southern California Road Cycling Club cyclists (CYC, n = 12). The race/ethnicity background of the athletes in RUN and CYC were predominantly European-White and Hispanic. A group of 12 physically active non-athletes from the Department of Kinesiology and Health Promotion at California State Polytechnic University, Pomona matched for age, weight and ethnic background with the athletes was recruited to serve as comparison group (CON). The RUN and CYC athletes were engaged in regular (10 out of 12 month per year) distance running or regular (10 out of 12 months per year) road cycling training/competition for a minimum of 5 years with an average training volume of 8 hr per week and at least 2 hours of running and 4 hours of cycling a day, 5-6 days a week. None of the athletes took more than 2 successive months per year off from training or competition. This information was obtained via a self-reported Health and Exercise History Questionnaire (HEHQ). The self-reported HEHQ also revealed that the RUN did not cross-train in cycling, and the CYC did not cross-train in distance running. The criteria for serving as the non-athlete control group included 1) participate in no more than 2 hr a week of physical activity for the previous 3 years, and 2) did not participate in any road cycling and distance running training or any other modality of athletic training such as soccer, tennis, badminton, volleyball and basketball in the previous 5 years. The exclusion criteria for serving as study subjects include: 1) diagnosed with a cardiovascular and pulmonary disease, diabetes, kidney and liver disease, orthopedic disorders, thyroid dysfunction, congenital or acquired bone disease, gastrointestinal disease (e.g. Crohn’s disease, irritable bowel syndrome) and cancer; 2) chronic use of prescribed medication or recreational drug such as anabolic steroid; 3) use of dietary supplements to enhance physical performance, 4) use of any tobacco product or alcoholic beverage; 4) body mass index (BMI) > 30 kg/m2 ; and 5) weight gain or loss of more than 10% of body weight over the past 3 months. The athletes’ sport training history in terms of age started training, number of years in training, and weekly training hours were obtained from the HEHQ.
Procedures: The measurement outcomes include height, weight, t ibial bending strength and calcaneus (heel) BMD, ulnar bending strength and distal radius and ulna (wrist) BMD, and maximal oxygen uptake. The procedures used in this study adhere to the principles of the Declaration of Helsinki. Prior to inclusion in the study, all subjects signed a written informed consent approved by the Institutional Review Board of Cal Poly Pomona. All measurements were conducted at the Musculoskeletal Research Laboratory of Cal Poly Pomona. All subjects underwent the following assessments: 1) anthropometric measures of height, weight, ulnar and tibial lengths, and ulnar width; 2) bone bending stiffness measurement of the ulna and tibia; 3) BMD scan of the wrist and heel; and 4) maximal oxygen uptake (VO2max) using a cycle ergometer.
Study measurements:
Anthropometric measurements: The subject reported to the Musculoskeletal Research Laboratory and was instructed to remove all metal objects from their arm and leg as well as shoes and socks. The subject’s weight (kg) was determined using a calibrated physician scale (Detecto, Webb City, Mo) and height (m) using a stadiometer (Accustat Genentech Stadiometer, South San Francisco, CA). Subjects’ body mass index (BMI = weight/ height2) was calculated from weight / height2 in kg/ m2. Their ulnar and tibial length were measured using metal tape in a sitting position. The ulnar and tibial length were used to calculate the ulnar and tibial bending strength.
Bone bending strength measurement: Subject’s bone bending strength was measured using a “Mechanical Response Tissue Analyzer” (MRTA) previously described [12,14,17]. The MRTA was originally invented at Stanford University and manufactured by Gaitscan Inc. (Gaitscan, NJ), and modified by NASA Ames Research Center, Life Science Division, Moffett Field, CA [20, 21]. Briefly, the instrument is a non-invasive in vivo technique that applies transcutaneous low-frequency random vibration to the mid-diaphysis of a long bone using an electromagnetic probe. The force and acceleration characteristics are then relayed to a signal analyzer and then to a computer equipped with a mathematical model for calculating bone bending stiffness. The mathematical model accounts for soft tissue mass, stiffness, and damping; skin damping; and bone mass, axial stiffness, and damping. The bone’s cross-sectional bending stiffness (EI in Nm2) is the product of young’s modulus of elasticity, E (a material property), and the cross-sectional moment of inertia, I (a structural property). Young’s modulus reflects the inherent stiffness of the bone; whereas the cross-sectional moment of inertia reflects bone geometry and is proportional to bone width (BW) of a cylindrical bone and expressed as: I α BW4. The cross-sectional bending stiffness of a long bone is expressed as: k = F/δmax = 48EI / L³, where F is the load applied at the midspan, k is the lateral stiffness of a beam in three-point bending, δm is the maximum bending displacement, and L is the length of the long bone [17, 20]. The MRTA technology uses the dynamic response of bone to calculate the lateral stiffness (k) to obtain EI. Thus, from the above equation EI = kL³ / 48. For this study, bone bending strength (EI) was obtained from the left tibia and left ulna. Quality control (QC) of the instrument was determined by performing daily measurement of an aluminum rod with a known EI value of 28.7 Nm2. All QC estimates were within 9% of the calculated EI [mean ± SD: 31.7 ± 1.2, and coefficient of variance (CV) = 3.8%]. Reproducibility was performed by repeated measures in the same individual and the CV of ulna EI was 3.2% and of tibial EI was 10.8%.
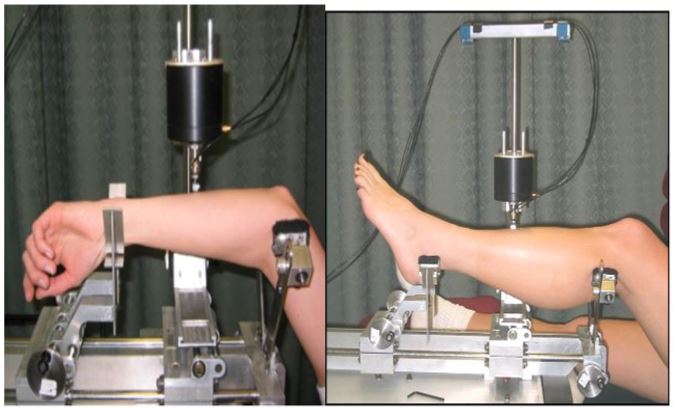
The ulnar bending strength assessment: The subject lies on the examination table with his head towards the MRTA instrument. The subject raised his left forearm above his head and rested the forearm epicondyles of the distal humerus on the top of the two small, padded metal cylinders, attached to the proximal supports of the MRTA instrument (figure 1a). The wrist, radial side down, is rested on the padded metal cylinders at the distal supports. The left forearm is placed so that the ulna is facing up, and the probe can be lowered and rested on the center of the ulna bone.The MRTA instrument automatically centers the probe between proximal and distal supports. A brief 10 second vibration is given, and the resonant response is recorded and analyzed using a 7-parameter algorithm computer soft-ware program. This assessment procedure was repeated 5 times. Each time, the operator evaluates the curve-fitting response using a 9-parameter algorithm [20]. The root means square error (RMSE) for the ulna EI tests on the same individual using the 9-parameter algorithm was between 3% and 7%.
The tibia bending strength assessment: For the tibial bending strength assessment, the subject lies on the examination table with his head aways from the MRTA instrument. The subject raises his left tibia (i.e., near the knee) and placed it on the proximal support and distal end of the tibia (the medial and lateral malleoli) on the distal supports so that the left tibia is suspended by the two supports (figure 1b). The height of the supports was adjusted so that the tibia is parallel to the base of the MRTA. A shaker probe is then lowered on to the middiaphysis of the subject’s tibia, vibrating it for approximately 10 seconds to record resonant responses. This procedure was repeated 5 times to obtain a total of 5 measurements on the same subject. Each time, the operator evaluated the curvef itting response using a 12-parameter improved by Roberts et al. (17). The RMSE for the tibia EI measurements on the same individual using the 12-parameter algorithm was between 7% and 9.3%.
Measurement of Bone Mineral Density: A Peripheral Instantaneous X-ray Imager (Lunar PIXI, GE Medical Division, Madison, WI), a dual-energy X-ray absorptiometry, was used to determine areal bone mineral density (BMD) of the heel and wrist. The measurements were performed on the left heel and left wrist. After removing all metal objects, shoes, and socks the subject was asked to sit and place his heel in a V-shape foot support with toes touching the opposite side of the V-shape support of the PIXI machine for the heel BMD measurements. The PIXI scanner takes 10-15 seconds to scan the heel BMD.
Figure 1: MRTA assessment of ulnar (a) and tibial (b) bending strength.
Figure 1: (a) Assessment of ulnar bending strength (EI) with the MRTA instrument. The subject lies down on a therapy treatment table, head toward the MRTA instrument, and raises the right forearm above the head, resting the epicondyles of the distal humerus on the top of the two small metal cylinders which are attached to the proximal supports of the MRTA instrument. The distal forearm is placed on the distal supports of the MRTA instrument so that the ulna is uppermost, and the magnetic shaker probe with the attached impedance head is lowered and rested on the center of the ulna. A brief 10 sec vibration is given by the magnetic shaker probe and the vibration resonant response is recoded and analyzed by the computer software program. The computer software program also calculates, and reports root mean square error (RMSE) for each of the 5 ulnar EI measurements on the same subject. The upper limit of an acceptable measurement for RMSE for each ulnar EI was set at <8%.
For statistical analysis, we used the average value of the subject’s ulnar EI measurements that was < 8%. (b) Assessment of tibial bending strength (EI) with the MRTA instrument. The subject lies down on a therapy treatment table, facing upward, head away from the MRTA instrument, and places the right tibia on the proximal supports and the distal end of the t ibia (the medial and lateral malleoli) on the distal supports so that the tibia is facing upward and suspended. Then, the magnetic shaker probe with the attached impedance head is lowered and placed on the center of the tibia, a brief 10 sec vibration is given by the magnetic shaker probe and the vibration resonant response is recoded and analyzed by the computer software program. The computer software program also calculates, and reports root mean square error (RMSE) for each of the 5 tibial EI measurements on the same subject. The upper limit of an acceptable measurement for RMSE for each tibial EI was set at <8%. For statistical analysis, the average value of the subject’s tibial EI measurements that was < 8% was used.
For the wrist BMD, the subject was asked to sit and gently hold a rounded object (a golf ball) to ensure correct position of the wrist for the PIXI scan. The PIXI scanner takes 10-15 seconds to scan for the wrist BMD. The printed X-ray image of the distal forearm was used to estimate ulnar width. A Kern caliper (Kern, Hartchrom, Switzerland) was used to measure the width (mm) of the ulna 30 mm from the junction of the ulna and radius. BMD of the heel and wrist were reported as g/cm2. The CV of the PIXI scanner measured BMD based on phantoms for the wrist was 3.45%. Based on repeated measures of the same subject, the CV of the BMD for the wrist was 1.2%.
Measurement of maximal oxygen uptake: Maximal oxygen uptake (VO2max ) was measured using an electronically braked cycle ergometer (Lode Excaliber, Groningen, The Netherlands) and an automated metabolic cart (Parvo TrueOne® 2400 Metabolic Cart, ParvoMedics, Sandy, UT). One day prior to measuring VO2max the subjects were asked to refrain from strenuous activity and alcohol consumption and were instructed to refrain from caffeine consumption the morning of the test. For the runners and the non-athletes, saddle height was adjusted so that the knee was slightly bent during the bottom of the pedal stroke. For the cyclists saddle height knee bend position were allowed to self-select to better reflect their ‘normal’ positioning on their own outdoor bicycle. All subjects wore toe straps during the test and were allowed to self-select the cycle work rate for the warm-up period (10-15 min) prior to beginning the maximal oxygen uptake test. Following the warm-up period, the subjects were outfitted with a nose-clip and a mouthpiece connected to a two-way valve (Hans Rudolph 2700 series, Kansas City, MO). The subjects then began a “ramp-type” cycle protocol beginning at 0 Watts (W) and continuously ramping the work rate by 25 W per min or 5 W every 12 seconds for the cyclists, and 20 W per min or 4 W every 12 seconds for the runners and non-athletes. Pedaling cadence was self-selected within a range of 60 – 80 rpm. During the maximal oxygen uptake test the following variables were recorded: maximal work output in peak W and average W, maximal heart rate and rating of perceived exertion (RPE) using the Borg 6-20 scale. Maximal oxygen uptake was determined when the subject met at least two of the following conditions during the maximal cycle exercise test: 1) a leveling off of VO2 with increasing work rate (i.e., increase < 2 ml · kg-1 · min-1), 2) respiratory exchange ratio > 1.10, 3) heart rate > 90% of predicted maximal heart rate using 220-age formula,
4) failure to maintain the prescribed pedaling rate for > 5 seconds despite continuous verbal encouragement, 5) rating of perceived exertion (RPE) higher than 19 (Borg 6-20 scale), or 6) volitional fatigue. Value of the VO2max was expressed as ml/kg/ min.
Data Analysis
Outcome variables were analyzed using a one-way analysis of covariance (ANCOVA). The covariates were height, weight and training history (recorded in year). When significant differences are found, Tukey’s HSD post hoc test was used to locate the source of difference. A robust test of equality of the means was performed using W procedure. A sample size was determined by power analysis based on a one-way analysis variance. We set the alpha at .05, a power of 0.80, and calculated the effect size (ES) according to Thomas and Nelson [22]. For the wrist and heel BMD, the ES of 1.13 and 1.57 were obtained, respectively. Based on these ES values, a sample size of 10 per group would be needed to detect significant differences between the study groups. For comparison of the ulnar (EI) and tibial (EI) bending strength between the CYC and RUN, a sample size of 15 per group each for the RUN and CYC, respectively, would be needed. With the sample size of 12 CYC and 12 RUN, the statistical power of .70 was obtained in this study. Statistical significance was set at p < 0.05.
Results
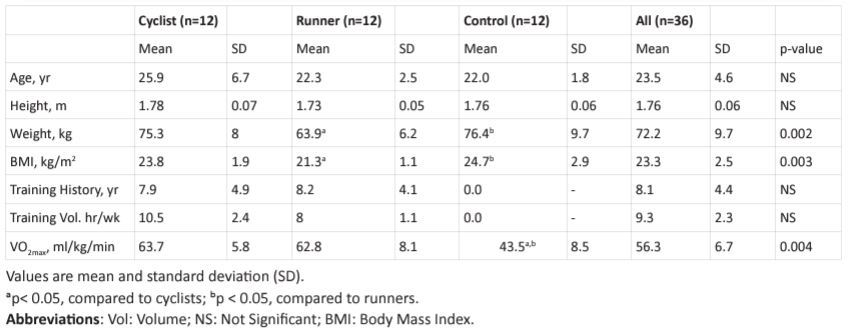
Physical characteristics and training history: Table 1 depicts
Table 1: Physical and physiological characteristics, training history and weekly training volume of the cyclists, runners, and non-athletes.
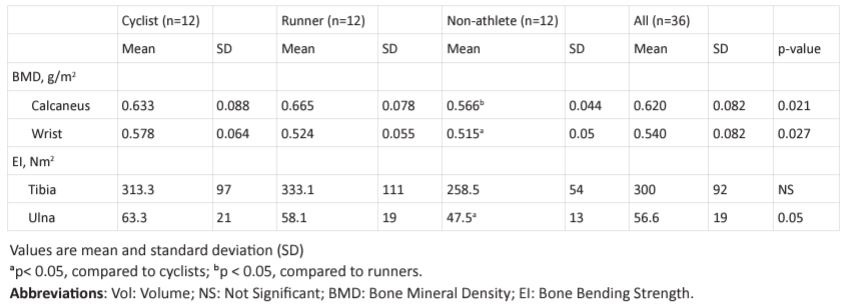
Table 2: BMD and bone bending strength of the cyclists, runners and non-athletes.
subjects’ physical characteristics, training history and VO2max . There was no statistical difference between the three groups in age and height. In terms of weight, the CYC and CON were 15.1% and 16.4%, respectively, heavier (p< 0.05) than RUN, but not between CYC and CON. Training history in terms of year of training and training volume in terms of hours per week were nearly identical between CYC and RUN. For example, the number of years of training in RUN and CYC were 8.9 and 8.0 years, respectively, and the training volume in RUN and CYC were 8 and 10.5 hr/week, respectively. The RUN began training at age 13.9 years which was significantly (p< 0.05) earlier than CYC at 18.5 years. Table 2 shows that tibial EI of CYC was 8% lower than RUN and 12.6% higher than CON. Differences in t ibial EL between the RUN and CYC were not significance. Ulnar EI of CYC was 7.4% higher than RUN and 27.9% higher than CON (both p < 0.05). Results also show that ulnar width were not significantly different between RUN, CYC and CON. Calcaneus BMD of the CYC was 5.2% lower than (p < 0.05) RUN and 8.5% higher than (p < 0.05) CON. Calcaneus BMD of RUN was 13% higher (p < 0.05) than CON. Wrist BMD of the CYC was 9.5% higher than RUN and 8.9% higher than CON (both p> 0.05).
Maximal oxygen uptake (VO2max ): VO2max (ml/kg/min) of the RUN and CYC were near identical and were 30.7% and 31.9%, respectively, higher (p < 0.05) than CON.
Discussion
The novel feature of the present study is the application of a direct non-invasive measurement of bone bending strength (i.e., EI in Nm2) by applying the MRTA technology to differentiate tibial and ulnar bending strength between male athletes chronically engaged in training and competition in road cycling or distance running. Myburgh et al. [23] reported in adult men who trained the upper limbs which comprised of non-weight bearing bone more than 6 h a week developed higher ulna bending strength than those who did not trained the upper limbs. Liang and colleagues using the MRTA instrument and reported that welltrained female synchronized swimmers developed ulnar and t ibial bending strength similar to well-trained female gymnasts [12]. The MRTA is an in vivo dynamic mechanical tissue response test that uses a vibration response analysis technique to make direct functional measurement of the mechanical properties (mass, stiffness, and damping) of long bones in humans and has the ability to detect large changes in bone strength that are undetected by bone densitometry [16]. According to Rubin and colleagues mechanical strain, induced noninvasively in the high frequency domain, is anabolic to cancellous bone but not cortical bone [24]. The other novel finding from the present study is that the results show similar bending strength in the t ibial and ulnar bones between CYC and RUN notwithstanding the differences in musculoskeletal loading histories and activities between the two sports. Liang and colleagues found more than double in bending strength in the ulna and tibia of well-trained female synchronized swimmers and gymnast than untrained females [12]. Compared to the non-athletes, the difference in tibial and ulnar EI between the CYC and RUN was a 22 - 33% and 21 - 29% higher in bending stiffness in the ulna and tibia, respectively. Courteix and colleagues suggested that training initiated during the high school years as teenagers and continued at high-intensity level to college years as adults accounted for exhibiting greater bone strength in the welltrained athletes [25]. However, Taafe and colleagues found that earlier training beginning at the high school years did not influence BMD outcomes in collegiate swimmers and runners [7]. Study results supported the hypothesis that CYC would have higher ulnar bending strength and wrist BMD than nonathletes (Table 2). This outcomes observation may be attributed to the repetitive low-magnitude, high-frequency vibration experienced during CYC training and competition while holding on to the handlebar during long hours road cycling and not during long-distance running and competition. The observation is substantiated by Rubin’s study who exposed animal hind limbs to 20 min/day of low-magnitude (0.3 g), high-frequency (30 Hz) mechanical vibrational stimulus, 5 days a week for one year and found a 34% increase in BMD in the skeleton subjected to vibration stimulus [24]. The adaptive response of long bone was localized and the potential anabolic response of the highfrequency signal was evidenced only in trabecular bone, not the cortical region of the long bone [24]. The MRTA technology for measurement of ulnar bending strength is an assessment of long bone which consists of both cortical shell and trabecular envelop of the ulna or tibia. Rubin and colleagues suggested that low-magnitude, high-frequency mechanical vibration signals generated during cycling activity at the forearm may be responsible for bone adaptation and remolding [24]. Note that the CYC exhibited significantly greater wrist BMD and ulnar bending strength than NA, not RUN (Table 2). Results support the hypothesis that RUN have higher calcaneus BMD than NA, not CYC. Giddings and colleagues reported that during running large magnitude of forces and calcaneal stresses are generated late in the stance phase, with maximum loads occurring at about 60% of the stance phase [26]. During running, the predicted peak talocalcaneal and calcaneocuboid joint loads is 3.9 and 7.7 body weight (BW), respectively [26]. Road cycling exerts tensile force initiated from lower limb muscle contraction at the skeleton it attached to induces negligible impact loading force on the lower limb skeletal tissue [12]. Matsumoto and colleagues observed that calcaneus BMD of collegiate male long-distance runners retained denser bone mineral than male Judo athletes and swimmers [27]. Researchers have reported that the adaptive potential of bone is responsive to long-term high-impact exercises such as running, gymnastic, step-aerobic, and resistance training [12, 28]. This is because bone tissue has the ability to rapidly accommodate changes in its mechanical environment to ensure sufficient growth in skeletal mass to support the load placed upon them [29]. A possible osteogenic mechanism for increasing bone mass with musculoskeletal loading from chronic distance running and road cycling could be associated with mechanical induce intracellular nitric oxide (NO) production of the cell body [30,31]. Delgado-Calle and colleagues elaborated that via pulsating fluid flow to extracellular space on the canaliculi and lacunae system of bone induced by mechanical stimulation that initiates intracellular nitric oxide production of the cell body [32]. NO is a known mediator of the response of osteocytes to mechanical loading and it mediates the induction of bone formation [31, 33]. The gains in tibial bending strength and BMD of calcaneus in RUN compared to NA could be due to the release of NO which is a known mediator of the response of osteocytes to mechanical loading [30, 31]. The other possible mechanism for inducing osteogenic response is the regulation of bone remodeling and bone cell expression via the endocrine system by elevating serum testosterone and growth hormone [34, 35]. Our study has limitation that must be addressed. This cross-sectional study with low number of subjects per group (i.e., CYC = 12 and RUN = 12) and a power of .7 is limited for drawing any conclusion on causal relationship.
Conclusion
The conclusion is that chronic engagement in high volume road cycling training induces osteogenic response for increased ulnar bending strength and chronic engagement in high volume long-distance running induces high calcaneus BMD in men. There are no differences in tibial bending strength and calcaneus BMD between distance runners and road cyclists. Further research is warranted to investigate differences in distance running and road cycling by incorporating upper body strength training to assess the outcomes on ulnar bending strength and wrist/forearm BMD.
Authors contributions: All authors contributed to the conceptions and design of the study. Materials preparation and data collection for BMD, bone bending strength, and VO2max were performed by MTCL, KL, ATA and JRR. Statistical analyses were performed by KL and MTCL. The first draft of the manuscript was written by MTCL, and all authors commented on all versions of the manuscript. All authors read and approved this final version of the manuscript.
References
- Vasikaran S, Cooper C, Eastell R, Griesmacher A, Morris HA, et al. International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med. 2011; 49: 1271-1274.
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A et al. Incidence and economic burden of osteoporosis-related fractures in the United States. 2007; 2005-2025. J Bone Miner 22:465-475.
- Nordstrom A, Karlsson C, Nyquist F, Olsson T, Nordstrom P, et al. Bone loss and fracture risk after reduced physical activity. J Bone Miner Res. 2005; 20: 202-207.
- Vincent-Rodriguez G. How does exercise affect bone development during growth? Sport Med. 2006; 36: 561-569.
- Gordon CM, Zemel BS, Wren TA, Leonard MB, Bachrach LK, Rauch F, et al. The Determinants of Peak Bone Mass. J Pediatr. 2017; 180: 261-269.
- Bonjour JP, Chevalley T, Rizzoli R, Ferrari S. Gene-environment interactions in the skeletal response to nutrition and exercise during growth. Med Sport Sci. 2007; 51: 64-80.
- Taaffe DR, Robinson TL, Snow-Harter CM, Marcus R. High impact exercise promotes bone gain in well trained female athletes. J Bone Miner Res. 1997; 12: 255-260.
- Vlachopoulos D. Barker AR, Williams CA, et al The impact of Sport Participation on Bone Mass and Geometry in Male Adolescents. Med Sci Sports Exerc. 2017; 49(2): 317-326.
- Zouch M, Vico L, Frere D, Tabka Z, Alexandre C. Young male soccer players exhibit additional bone mineral acquisition during the peripubertal period: 1-year longitudinal study. Eur J Pediatr. 2014; 173(1): 53-61.
- Vainionpää A, Korpelainen R, Leppäluoto J, Jämsä T. Effect of high impact exercise on bone mineral density: a randomized controlled trial in premenopausal women. Osteoporos Int. 2005; 16: 191-197.
- Rocha-Rangel J, Liang MTC, Anderson Hwa-te Tsai, Alexandra T. Auslander, Patricia Robles, Yuan-Lieh Kwon, Sara B. Arnaud. Bone Bending Strength and BMD of Female Athletes in Volleyball, Soccer, and Long distance Running. Eur J Appl Physiol. Published online. 2023. https://doi.org/10/1007/s00421-023-05231-2
- Liang, MTC, SB Arnaud, CR Steele, P Hatch, A Moreno. Ulnar and Tibial bending stiffness as an Index of Bone Strength in Synchronized Swimmers and Gymnasts. Eur J Appl Physiol. 2005; 94(4): 400-407.
- Miller EL, Wootten DF, Nickols-Richardson SM, Ramp WK, Steele CR, Cotton JR, Carneal JP, Herbert WG. Isokinetic training increases ulnar bending stiffness and bone mineral in young women. Bone. 2007; 41(4): 685-689. Epub 2007 July 13zDOI: 10.1016/j.bone. 2007.07.004.
- Dutto DJ, Liang MTC, Braun WA, Bassin SL, Wong ND, Arnaud SB. Effect of 6-month high impact step aerobics and resistance training on BMD and tibial bending strength in sedentary premenopausal women. Osteoporos Int. Published online. 2021 https://doi:org/10.1007/s00198-021-06106-y. 2021.
- Hutchinson TM, Bakulin AV, Rakhmanov AS, Martin RB, Steele CR, Arnaud SB. Effects of chair restraint on the strength of tibia in rhesus monkeys. J Med Primatol. 2002; 30: 313-321.
- Loucks AB, Clark BC, Bowman L, Response to “Clinical Evaluation of Bone Strength and Fracture Risk”. Curr Osteoporos Rep. 2017; 15(4): 396-397. Doi: 10.1007/s11914-017-0386-8.
- Roberts SG, Hutchson TM, Arnaud SB, Kirati BJ, Martin RB, Steele CR. Noninvasive determination of bone mechanical properties using vibration response: a refined model and validation in vivo. J Biomech 1996: 29(1): 91-98.
- Nikander R, Kannus P, Rantalainen T, Uusi-Rasi K, Heinonen A, Sievänen H. Cross-sectional geometry of weight-bearing tibia in female athletes subjected to different exercise loadings. Osteoporos Int. 2010; 21: 1687-1694. https://doi.org/10.1007/s00198-009-1101-0.
- Nikander R, Sievanen H, Uusi-Rasi K, Heinonen A, Kannus P. Loading modalities and bone structures at nonweight-bearing upper extremely and weight-bearing lower extremity: A pQCT study of adult female athletes. Bone. 2006; 39: 886-894.
- Steele CR, Zhou LJ, Guido D, Marcus R, Heinrichs WL, Cheema C. Noninvasive determination of ulnar stiffness from mechanical response – in vivo comparison of stiffness and bone mineral content in humans. J Biomech Engng. 1988; 110: 87-96.
- Arnaud SB, Steele CR, Zhou L-J, Hutchinson T, Marcus R. A direct non-invasive measure of long bone strength. Proc Ann Int Cong IEEE Eng Int Med Biol Soc. 1991; 13: 1984-1985.
- Thomas JR, Nelson JK. Research Methods in Physical Activity, Fourth Edition, Human Kinetics: Champaign, IL. 2001; 135-144.
- Myburgh K, Charette S, Zhou L-Z, Steele CR, Arnaud SB, Marcus R. Influence of recreational activity and muscle strength on ulnar bending stiffness in healthy men. Med Sci Sports Exerc. 1993; 25: 592-596.
- Rubin C, Yurner AS, Mallinckrodt C, Jerme C, McLeod K, Bain S. Mechanical strain, induced noninvasively in the high frequency domain, is anabolic to cancellous bone, but not cortical bone. 2002; 30: 445-452.
- Courteix D, Lespessailles E, Peres SL, Obert P, Germain P, Benhamou CL. Effect of physical training on bone mineral density in prepubertal girls: a comparative study between impact-loading and non-impact-loading sports. 1998; 8(2): 152-158. doi: 10.1007/BF02672512.
- Giddings VL, Beaupré GS, Whalen RT, Carter DR. Calcaneal loading during walking and running. Med Sci Sports Exerc. 2000; 32(3): 627-634.
- Matsumoto T, S Nakagawa, S Nishida, and R Hirota. Bone density and bone metabolic markers in active collegiate athletes: findings in long-distance runners, judoists, and swimmers. Int. J Sports Med. 1997; 18(6): 408-412.
- Snow-Harter C, Bouxsein M, Lewis B, Carter D, Marcus R. Effects of resistance and endurance exercise on bone mineral status of young women. J bone Miner Res. 1992; 7: 761-769.
- Frost H. Skeletal structural adaptations to mechanical usage. Anat Res 1990; 26: 403-413.
- Vatsa A, Mizuno D, Smit TH et al. Bio imaging of intracellular NO production in single bone cells after mechanical stimulation. J Bone Miner Res. 2006; 21: 1722-1728.
- Klein-Nulend J, van Oers RFM, Bakker AD, Bacabac RG. Nitric oxide signaling in mechanical adaptation on bone. Osteoporos Int. 2014; 25: 1427-1437. DOI 10.1007/s00198-013-2590-4
- Delgado-Calle J, Riacho JA, Klein-Nulend J. Nitric oxide is involved in the down regulation of SOST expression induced by mechanical loading. Calc Tis Int. 2014; 94(4): 373-383.
- Nitric-Oxide in bone and joint disease edited by Hukkanen MVJ, JM Polak, and SPF Hughes. Cambridge: Cambridge University Press. 1998.
- Bosco C, Lacovelli M, Tsarpela O, Cardinale M, Bonifazi M, Tihanyi J, Viru M, De Lorenzo A, Viru A. Hormonal responses to whole-body vibration in men. Eur J Appl Physiol. 2000; 81(6): 449-454. Doi:10.1007/s004210050067.
- Kvorning T, Bagger M, Caserotti P, Madsen K. Effects of vibration and resistance training on neuromuscular and hormonal measures. Eur J Appl Physiol. 2006; 96(5): 615-625.